What is the atomic number of palladium?
45
46
47
48
Dive into the world of Palladium with our comprehensive guide, designed to enlighten enthusiasts and professionals alike on this precious metal’s invaluable uses and characteristics. This introduction offers a detailed exploration into Palladium’s applications, from its critical role in automotive catalytic converters to its burgeoning presence in electronics and jewelry. With practical examples, we unpack the versatility and economic significance of Palladium.
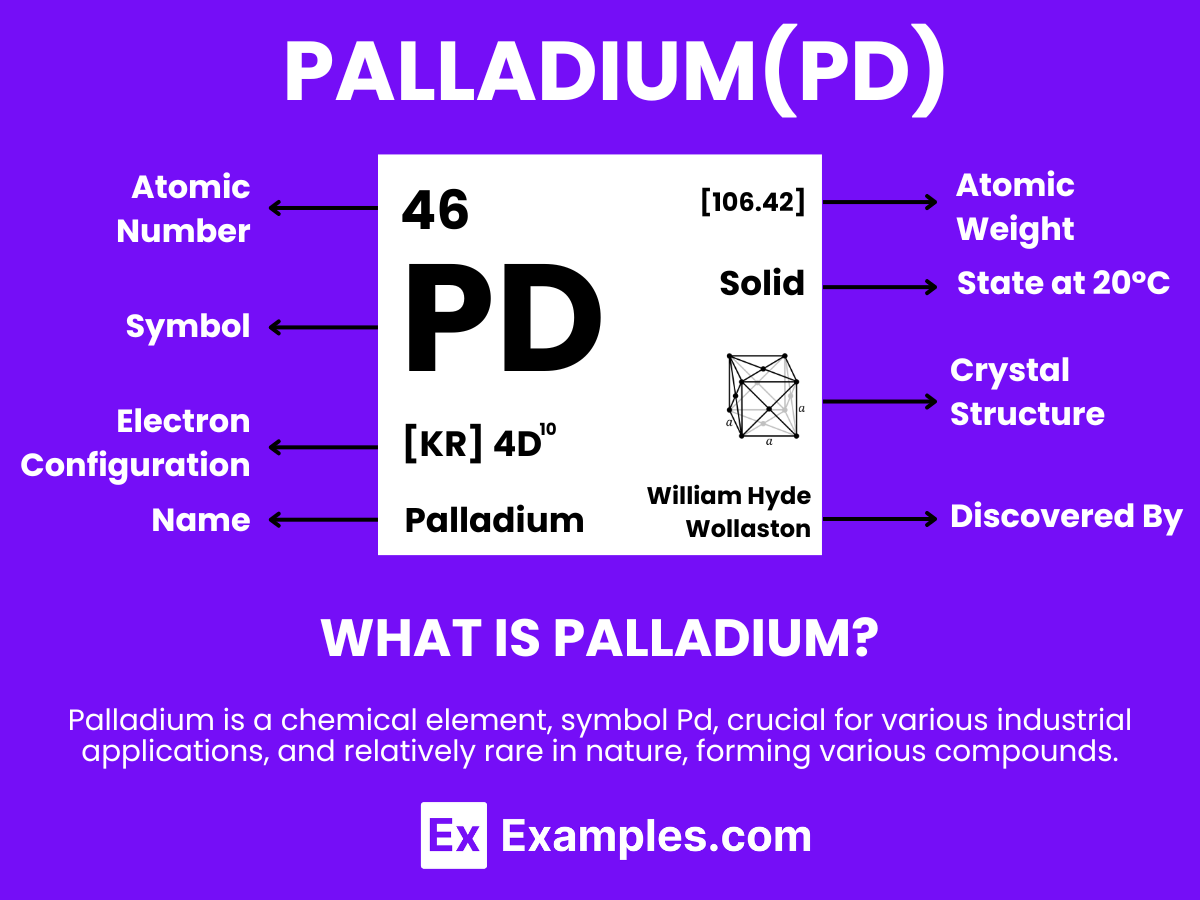
Palladium is a lustrous, silvery-white metal that distinguishes itself through its unique properties and wide range of applications. With the atomic number 46. Palladium is noteworthy for its excellent ability to absorb hydrogen, making it an essential material in hydrogen storage and purification technologies. This element is not found as a free metal in nature but is extracted from nickel and copper ores. Additionally, its exceptional catalytic properties make it indispensable in the fields of electronics, dentistry, and jewelry making. Its capacity to form alloys with other metals further amplifies its utility, positioning Palladium as a vital component in scientific research, environmental technologies, and the advancement of cutting-edge innovations
Palladium, with its atomic number of 46, stands out in the periodic table due to its unique atomic structure and impressive properties. This lustrous, silver-white metal has an atomic weight of 106.42 u and belongs to the platinum group metals, characterized by its remarkable resistance to corrosion and oxidation at room temperature.
The electron configuration of palladium is [Kr] 4d¹⁰ , making it one of the few elements with all of its d-orbitals filled, a trait that contributes to its exceptional catalytic abilities. In its solid state, palladium adopts a face-centered cubic (fcc) crystal structure, which is pivotal for its application in various catalytic and electronic devices. This dense, malleable metal finds extensive use in the automotive industry, electronics, and jewelry, primarily due to its atomic structure that facilitates a wide range of chemical reactions and enhances alloy formation.
| Property | Value |
|---|---|
| Atomic Number | 46 |
| Atomic Mass | 106.42 amu |
| Density | 12.023 g/cm³ at 20°C |
| Melting Point | 1,555°C (2,831°F) |
| Boiling Point | 2,963°C (5,365°F) |
| State at Room Temperature | Solid |
| Appearance | Silvery-white metallic |
| Crystal Structure | Face-centered cubic (fcc) |
Palladium, a noble metal, exhibits several unique chemical properties that make it invaluable in industrial applications, particularly in catalysis and electronics. Below are key chemical properties of Palladium, accompanied by relevant equations:
| Property | Value |
|---|---|
| Heat of Fusion | 16.74 kJ/mol |
| Heat of Vaporization | 357 kJ/mol |
| Specific Heat Capacity | 25.98 J/(mol·K) |
| Thermal Conductivity | 71.8 W/(m·K) |
| Thermal Expansion | 11.8 µm/(m·K) at 25°C |
| Property | Value |
|---|---|
| Atomic Number | 46 |
| Atomic Mass | 106.42 amu |
| Density | 12.023 g/cm³ at 20°C |
| Melting Point | 1,555°C (2,831°F) |
| Boiling Point | 2,963°C (5,365°F) |
| Crystal Structure | Face-centered cubic (fcc) |
| Hardness | 4.75 Mohs |
| Malleability | High, especially when annealed |
| Property | Value |
|---|---|
| Electrical Resistivity | 10.8 nΩ·m at 20°C |
| Magnetic Ordering | Paramagnetic at 300 K |
| Superconducting Temperature | Below 9 K (when alloyed) |
| Property | Value |
|---|---|
| Isotopes | Naturally occurring: ¹⁰²Pd, ¹⁰⁴Pd, ¹⁰⁵Pd, ¹⁰⁶Pd, ¹⁰⁸Pd, ¹¹⁰Pd |
| Most Stable Isotopes | ¹⁰⁶Pd (half-life: stable), ¹⁰⁸Pd (stable) |
| Neutron Cross Section | 6.9 barns (for ¹⁰⁵Pd) |
| Atomic Radius | 137 pm |
Palladium Chloride is used in catalysis and preparing palladium-based compounds, soluble in water.
Facilitates organic reactions as a catalyst, especially in C-C coupling processes.
Used in sensors and as a catalyst for hydrogen production and oxidation reactions.
Employed in purification processes and as a precursor for other palladium compounds.
Rarely used, but serves in specialized organic synthesis and catalysis research.
A catalyst for cross-coupling reactions, important in pharmaceuticals and organic electronics.
Palladium, a lustrous white metal in the platinum group, possesses various isotopes that contribute to its significance in science and industry. Below is a detailed table showcasing the most notable isotopes of palladium:
| Isotope | Atomic Mass | Natural Abundance (%) | Half-life | Mode of Decay |
|---|---|---|---|---|
| Pd-102 | 101.905609 | 1.02 | Stable | – |
| Pd-104 | 103.904036 | 11.14 | Stable | – |
| Pd-105 | 104.905085 | 22.33 | Stable | – |
| Pd-106 | 105.903486 | 27.33 | Stable | – |
| Pd-108 | 107.903892 | 26.46 | Stable | – |
| Pd-110 | 109.905153 | 11.72 | Stable | – |
| Pd-103 | 102.904579 | Synthetic | 17 days | Beta decay |
| Pd-107 | 106.905097 | Synthetic | 6.5 million years | Beta decay |
| Pd-109 | 108.904752 | Synthetic | 13.7 hours | Electron capture |
These isotopes play a crucial role in both academic research and practical applications, ranging from nuclear medicine to environmental studies.
Palladium, a versatile metal, is prized for its various applications across numerous fields. Below, we explore the key uses of palladium:
Palladium is mainly sourced as a byproduct from nickel and copper mining. The process starts with crushing the ore and treating it with water to create a slurry.
The slurry undergoes flotation to concentrate palladium-containing minerals, separating them from other materials.
Through smelting and leaching, the palladium is further concentrated, preparing it for the final purification steps.
Palladium is purified to high purity levels through electrolytic refining or solvent extraction techniques, making it ready for commercial use.
Palladium is extensively used in catalytic converters to reduce harmful emissions from vehicles, making it crucial for environmental protection.
It is employed in manufacturing electronic components like multilayer ceramic capacitors, enhancing device performance and durability.
As a catalyst, palladium facilitates hydrogenation and dehydrogenation reactions, playing a vital role in creating various chemicals and pharmaceuticals.
Due to its biocompatibility and aesthetic appeal, palladium is used in dental fillings and high-quality jewelry.
Palladium’s unique ability to absorb hydrogen makes it ideal for purification and storage applications, contributing to energy solutions.
palladium’s journey from its extraction as a byproduct of mining to its diverse applications showcases its indispensable role across various industries. From catalyzing reactions in automotive converters and chemical manufacturing to enhancing the quality of electronics, dental materials, and jewelry, palladium proves to be a versatile and valuable metal. Its unique properties not only contribute to technological advancements but also to environmental sustainability and aesthetic craftsmanship.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of palladium?
45
46
47
48
What is the symbol for palladium?
Pd
Pt
Pb
Pu
Which of the following properties is true about palladium?
High density
Poor conductor of electricity
Low melting point
Highly reactive
What is the primary use of palladium in industry?
Jewelry
Catalysts
Electronics
Batteries
Which mineral is the main source of palladium?
Chalcopyrite
Galena
Pentlandite
Cassiterite
What is the melting point of palladium?
961°C
1064°C
1554°C
1768°C
Palladium is most commonly found in which type of geological deposits?
Sedimentary
Igneous
Metamorphic
Alluvial
Which country is one of the largest producers of palladium?
Australia
Russia
Brazil
China
What is the density of palladium?
8.5 g/cm³
10.5 g/cm³
12.0 g/cm³
12.5 g/cm³
In which year was palladium discovered?
1803
1812
1824
1836
Before you leave, take our quick quiz to enhance your learning!

