Propane belongs to which homologous series?
Alkenes
Alkanes
Alkynes
Aromatics


Propane is a fascinating substance that plays a big role in our daily lives, often without us even realizing it! Think of it as a type of fuel that comes from natural gas and petroleum. This colorless, odorless gas is given a special smell so we can detect it easily if there’s a leak. In chemistry, it’s known as one of the simplest hydrocarbons, meaning it’s made up of hydrogen and carbon atoms arranged in a straightforward way. Its molecular formula is C₃H₈, indicating it has three carbon atoms and eight hydrogen atoms. Propane is versatile, used not just for heating homes and cooking food, but also in refrigerators, as a fuel for engines, and in making plastic! Understanding propane helps us appreciate the vast world of molecular compounds and how they impact our everyday life.
Often known as consumer-grade propane, HD5 stands for “High Density, 5% maximum propylene.” It’s the most common type of propane you’ll find being used in residential and commercial settings, such as for heating homes, fueling gas grills, and running propane-powered vehicles. HD5 propane is preferred for these applications because it’s the purest form, ensuring efficient and safe consumption.
This type is used more for industrial and commercial purposes rather than residential. Commercial propane can contain a higher percentage of propylene compared to HD5 propane. Because of this, it’s not as ideal for residential heating or cooking, but it works well for large-scale applications that require propane’s properties but don’t need the high purity level of HD5.
| Property | Value |
|---|---|
| Formula | CH₃CH₂CH₃ |
| Hill Formula | C₃H₈ |
| Name | Propane |
| Alternate Names | Dimethylmethane, Freon 290, N-Propane, Petroleum Gas, Propyl Hydride, R-290 |
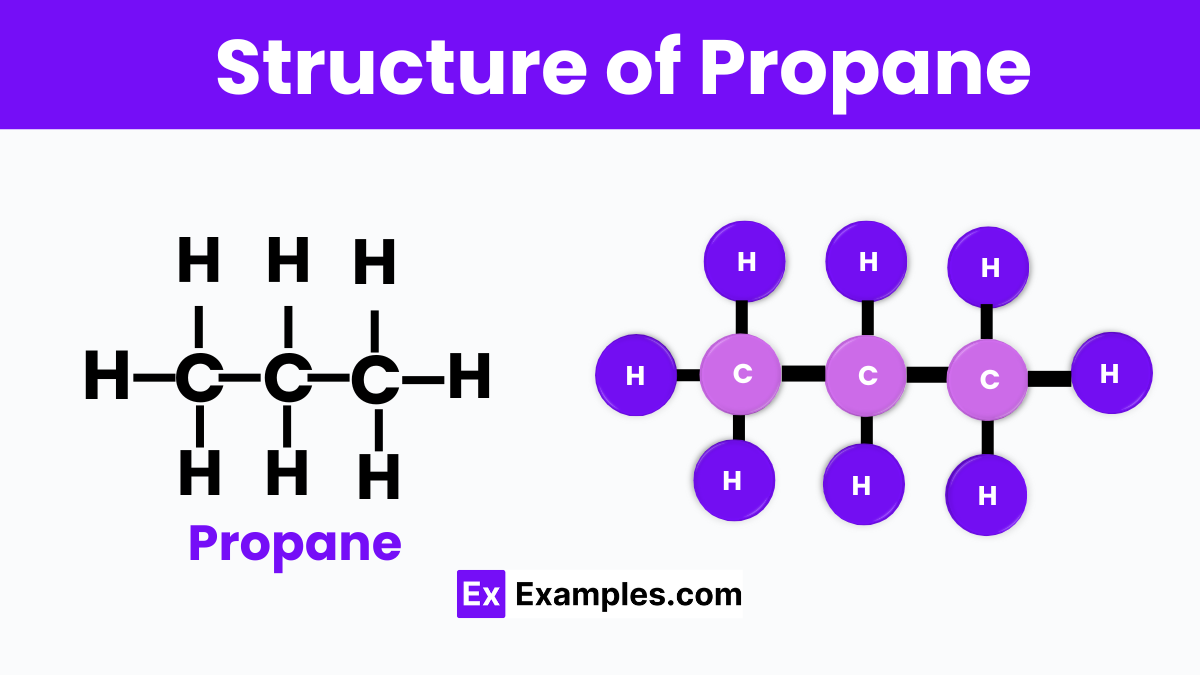
Imagine propane as a little train made up of three cars, where each car represents a carbon atom. This train, or molecule, is part of a larger family known as alkanes, which are made up solely of carbon and hydrogen atoms. In propane, the three carbon “cars” are linked together in a straight line, and each carbon atom is also connected to hydrogen atoms. Specifically, the carbon atoms at the ends of the train are each holding onto three hydrogen atoms, like balloons, while the middle carbon atom is holding onto two. This gives propane a total of eight hydrogen atoms, making its full chemical formula C3H8. This structure makes propane a gas at room temperature, but it can be easily stored as a liquid under pressure, which is why it’s so useful for fueling our barbecues, heating our homes, and even powering some vehicles.
Propane is created through a fascinating process that starts deep beneath the Earth’s surface. It’s primarily produced during the natural gas processing and petroleum refining. When crude oil is refined, or natural gas is processed, various hydrocarbons are separated out, and propane is one of these. The process involves cooling the gas, which turns the propane into a liquid so it can be easily separated and collected. This method is efficient and allows us to harness propane for everyday use.
Another interesting way to make propane in a lab setting involves a chemical reaction known as “cracking.” In this process, larger hydrocarbon molecules are broken down into smaller ones under high temperature and pressure. For example, when a larger molecule like hexane (C₆H₁₄) is cracked, it can produce propane (C₃H₈) and other smaller hydrocarbons. The chemical equation for this type of reaction could look something like this:
This equation shows hexane breaking down into propane and propylene (C₃H₆), a common byproduct. These methods of producing propane ensure that we have a steady supply of this valuable fuel for heating, cooking, and much more.
| Property | Description |
|---|---|
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless in its pure form; however, an odorant is added for safety to detect leaks |
| Boiling Point | -42°C (-44°F) |
| Melting Point | -188°C (-306°F) |
| Density | 0.51 kg/m³ at 0°C (32°F), making it lighter than air |
| Solubility in Water | Slightly soluble |
Propane is highly combustible, meaning it can catch fire and burn easily. This property makes it a valuable fuel source. When propane burns in the presence of oxygen, it produces carbon dioxide, water, and heat.
Equation: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O + heat
Propane reacts with halogens (like chlorine and bromine) in the presence of light or heat, forming halogenated compounds. This type of reaction is known as a substitution reaction because the hydrogen atoms in propane are replaced by halogen atoms.
Equation: C₃H₈ + Cl₂ → C₃H₇Cl + HCl
Propane is relatively stable under normal conditions but can react violently when mixed with oxidizing agents due to its combustible nature. This stability makes it safe to store and transport when guidelines are followed.
| Property | Value |
|---|---|
| CAS Registry Number | 74-98-6 |
| Beilstein Number | 1730718 |
| PubChem Compound ID | 6334 |
| PubChem Substance ID | 24857802 |
| SMILES Identifier | CCC |
| InChI Identifier | InChI=1/C3H8/c1-3-2/h3H2,1-2H3 |
| RTECS Number | TX2275000 |
| MDL Number | MFCD00009359 |
| Property | Value |
|---|---|
| NFPA Health Rating | 1 |
| NFPA Fire Rating | 4 |
| NFPA Reactivity Rating | 0 |

Many homes use propane for heating because it’s efficient and heats quickly. Unlike electric heaters, propane can warm up a space more evenly and reliably, even during power outages.
Propane is a popular choice for cooking, both indoors and outdoors. It provides instant heat and precise temperature control, making it a favorite for stoves, ovens, and barbecue grills.
Propane water heaters are known for their efficiency and speed. They can heat water faster and often cost less to operate than their electric counterparts, providing a continuous supply of hot water.
Propane refrigerators are essential in off-grid locations. They’re highly efficient and can operate without electricity, making them perfect for remote cabins or camping trips.
Propane is used as an alternative fuel for vehicles. Known as autogas when used in this way, it’s cleaner-burning than gasoline or diesel, reducing emissions and offering a cost-effective fuel option.
Propane generators are a reliable backup power source. They start easily in cold weather and, unlike gasoline, propane can be stored for a long time without degrading, ensuring you have power when you need it.
In agriculture, propane is used for drying crops and heating greenhouses. It’s a clean fuel option that helps protect the produce and provides a controlled environment for crop growth.
Industrially, propane is used in metalworking to fuel forges and furnaces. Its high combustion temperature makes it ideal for processes that require intense heat, like metal cutting and soldering.
Propane offers higher efficiency and portability than natural gas, making it ideal for areas without gas pipelines.
Natural gas is generally cheaper than propane due to its abundant supply and lower distribution costs.
Propane burns cleaner than other fossil fuels but still emits greenhouse gases, contributing to climate change.
Propane is a liquefied petroleum gas with a higher energy output, while regular gas is a liquid fuel used in vehicles.
Propane is safe for home use with proper installation and maintenance but poses risks of leaks and explosions if mishandled.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Propane belongs to which homologous series?
Alkenes
Alkanes
Alkynes
Aromatics
Which type of bond is predominant in propane?
Covalent bond
Ionic bond
Metallic bond
Hydrogen bond
What is the main use of propane in households?
As a solvent
For cooking and heating
For producing plastics
As a refrigerant
How many hydrogen atoms are present in a molecule of propane?
8
9
10
11
What is the boiling point of propane?
-42°C
0°C
36°C
100°C
In the propane molecule, how many sigma bonds are present?
7
8
9
10
Which process is used to obtain propane commercially?
Fractional distillation of crude oil
Electrolysis of water
Hydrogenation of ethene
Sublimation of dry ice
Propane can undergo which of the following reactions?
Addition reaction
Substitution reaction
Elimination reaction
Polymerization
Which catalyst is used in the cracking process to obtain propane?
Nickel
Platinum
Zeolite
Iron
The IUPAC name of propane is:
Propene
Propanol
Propane
Propyne
Before you leave, take our quick quiz to enhance your learning!

