What is the most stable form of carbon under standard conditions?
Graphite
Diamond
Fullerene
Carbon nanotubes
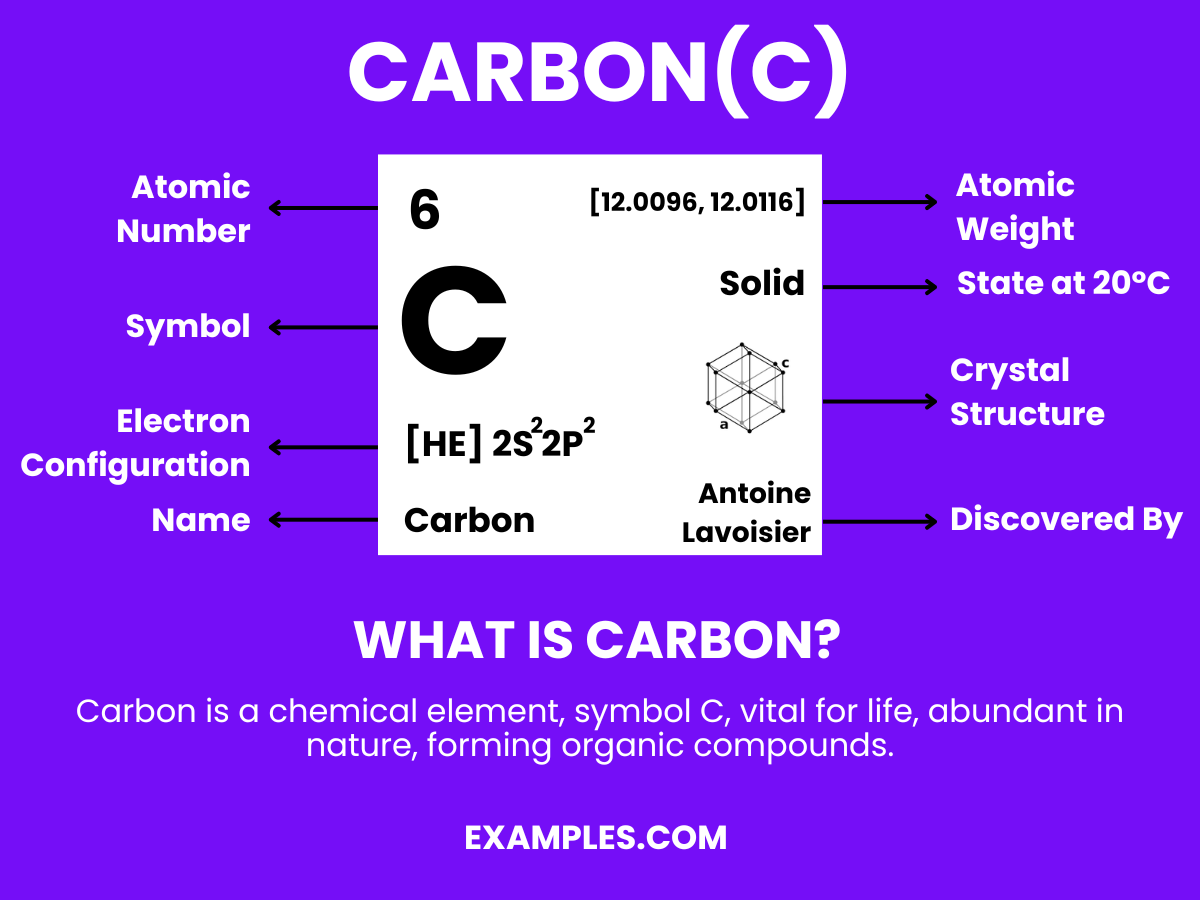
Discover the diverse world of Carbon, an essential element in both the cosmos and our daily lives. This complete guide illuminates Carbon’s role from the diamonds glittering beneath the earth’s surface to the vast array of organic compounds forming all known life. Dive into practical examples, understand its interaction with hydrogen in countless compounds, and grasp its significance in ecology, technology, and beyond. Embrace the carbon cycle with this enlightening exploration, enriched with engaging examples and essential tips.
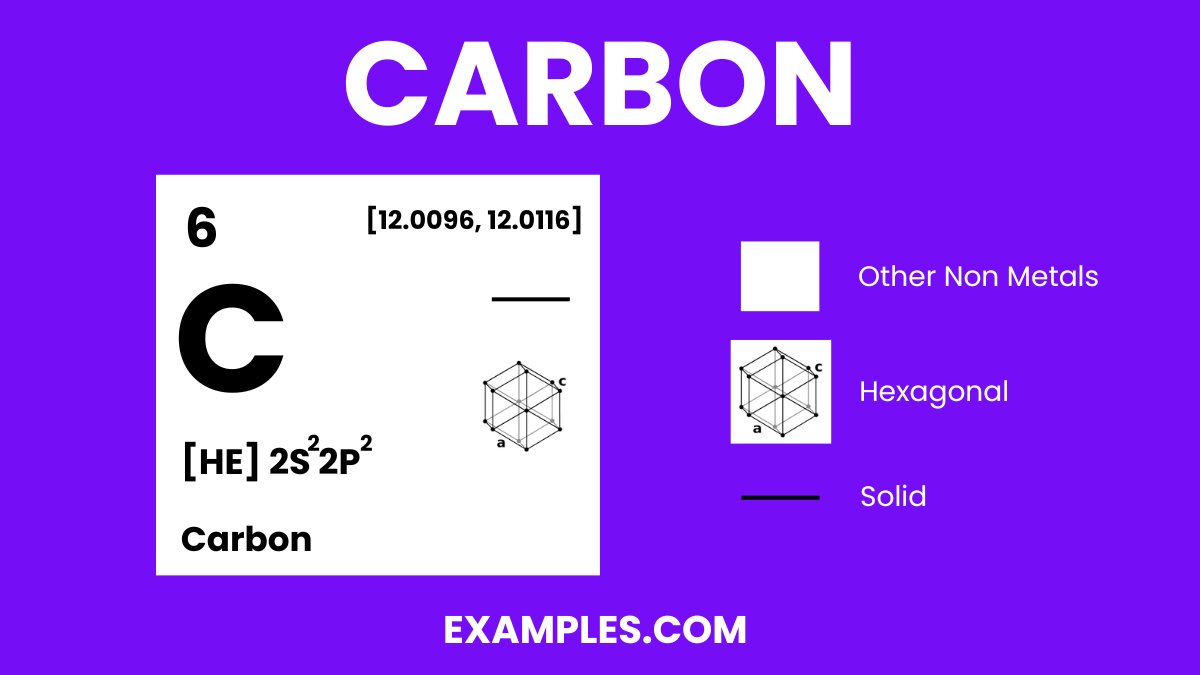
Carbon is a fundamental element, symbolized as ‘C’ on the periodic table, and is renowned for its versatility and abundance in both living organisms and the inanimate world. Known as the building block of life, carbon atoms form the backbone of organic chemistry, creating an immense variety of compounds when they bond with other elements, especially hydrogen, oxygen, and nitrogen. Its ability to form long chains and rings through bonding makes it unique, leading to an endless array of structures from simple gases like carbon dioxide to complex DNA molecules. Its allotropes, like diamond and graphite, showcase its diverse physical properties. Simple yet profound, carbon’s definition extends into every corner of science and daily life, making it an elemental cornerstone in education.
| Hydrogen | Sulfur |
| Nitrogen | Chlorine |
| Oxygen | Selenium |
| Fluorine | Bromine |
| Phosphorus | Iodine |
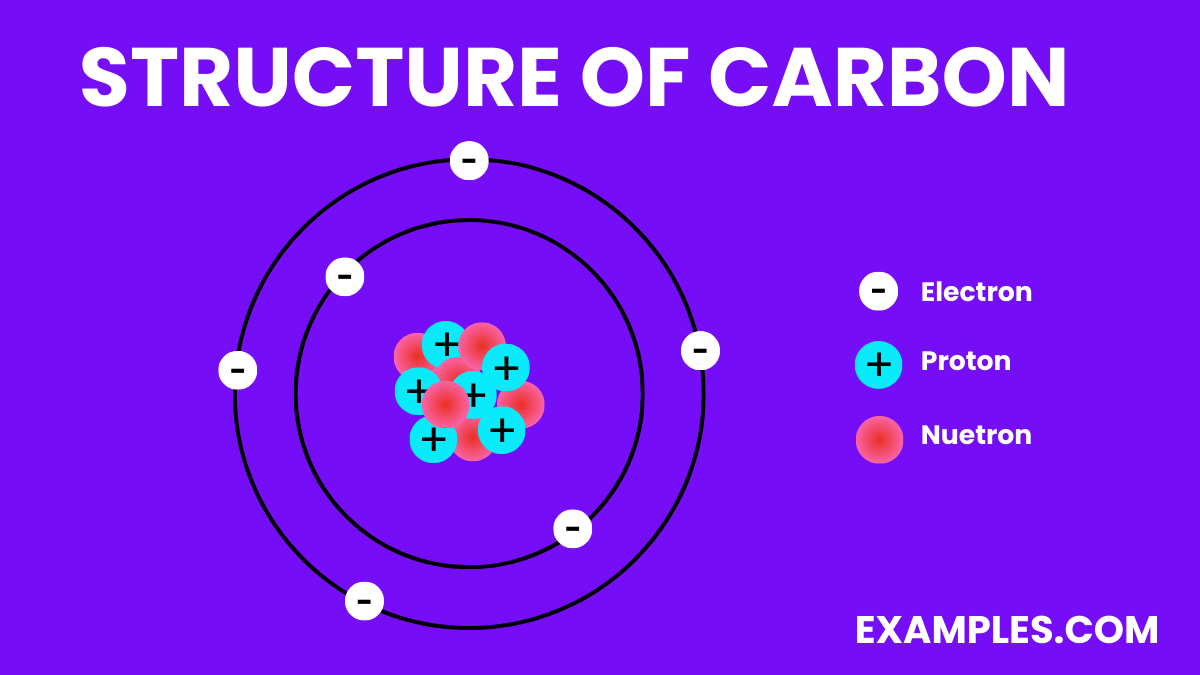
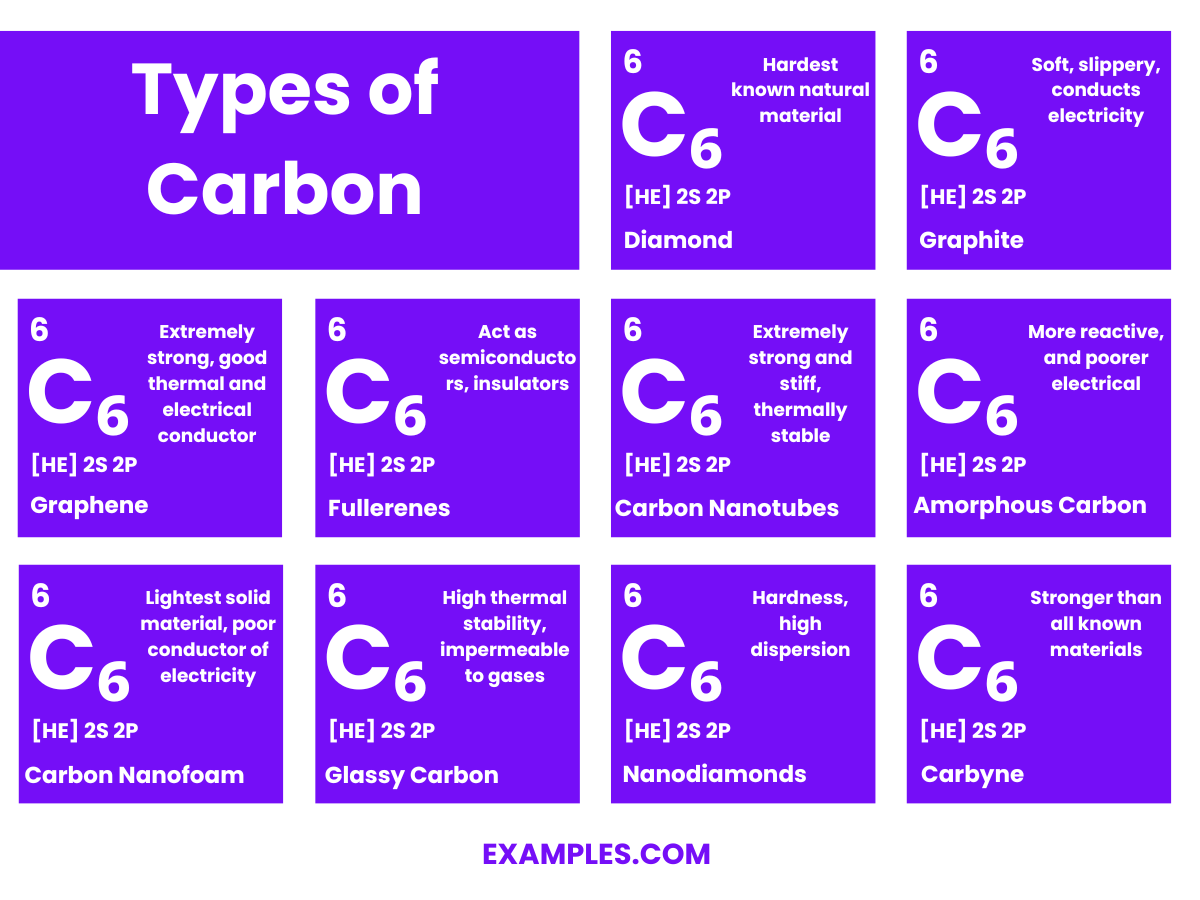
The structure of carbon varies significantly depending on its allotrope. Carbon is unique in its ability to form different structures (allotropes) due to its four valence electrons, which allow it to form strong covalent bonds with other carbon atoms in various arrangements. Here are some of the most well-known allotropes of carbon:
Each of these structures demonstrates the versatility of carbon in forming a variety of molecular configurations, leading to a wide range of physical and chemical properties. This versatility is what makes carbon so fundamental in chemistry, materials science, and biology.
| Property | Description |
|---|---|
| Allotropes | Exists as diamond, graphite, graphene, fullerenes |
| Color | Colorless as diamond, black as graphite |
| State at Room Temperature | Solid |
| Melting Point | 3550°C (diamond), Sublimes at ~3900°C (graphite) |
| Boiling Point | Sublimation point is ~3900°C (graphite) |
| Density | 3.51 g/cm³ (diamond), 2.267 g/cm³ (graphite) |
| Hardness | Hardest natural material (diamond), soft (graphite) |
| Electrical Conductivity | Insulator (diamond), Conductor (graphite) |
| Thermal Conductivity | Excellent in diamond, good in graphite |
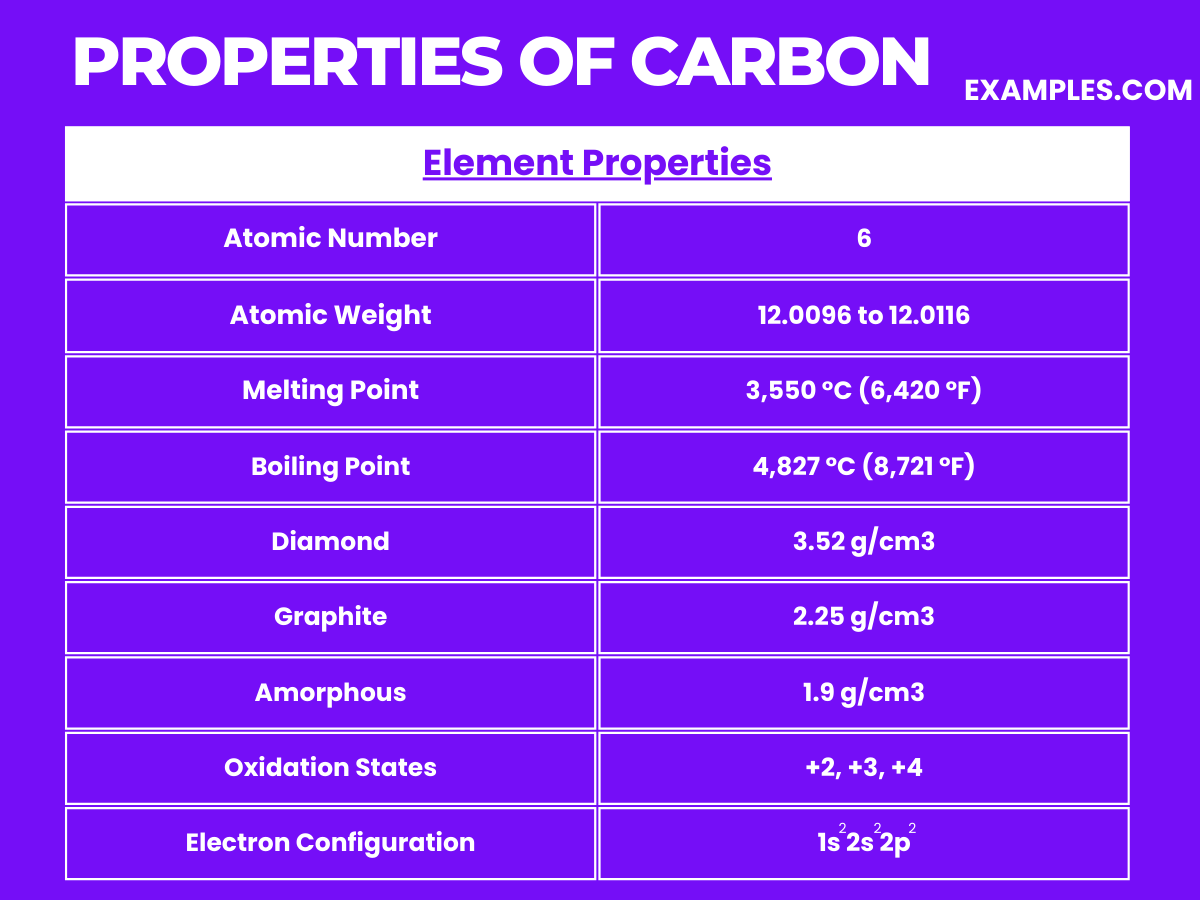
The physical properties of carbon make it an incredibly versatile element, suitable for a wide range of applications from industrial to technological and even in jewelry. Understanding these properties helps in comprehending the extensive use and significance of carbon in various fields.
These chemical properties, especially tetravalency and catenation, make carbon an essential element for forming the complex molecules necessary for life and a wide range of materials and chemicals used in various industries. The ability to form stable bonds with a wide variety of elements allows carbon to be at the center of organic chemistry.
| Property | Description / Value |
|---|---|
| Melting Point | Sublimation at 3915°C (graphite) |
| Boiling Point | 4827°C (graphite) |
| Thermal Conductivity | 119-165 W/(m·K) (diamond), 5-129 W/(m·K) (graphite) |
| Specific Heat | 0.71 J/(g·K) (diamond), 0.71 J/(g·K) (graphite) |
| Thermal Expansion Coefficient | 1 x 10^-6 /K (diamond), 8 x 10^-6 /K (graphite) |
| Property | Description / Value |
|---|---|
| Density | 3.51 g/cm³ (diamond), 2.267 g/cm³ (graphite) |
| Young’s Modulus | 1050 GPa (diamond), 8-15 GPa (graphite) |
| Tensile Strength | Variable depending on form |
| Mohs Hardness | 10 (diamond), 1-2 (graphite) |
| Elastic Modulus | 1,200 GPa (diamond) |
| Property | Description / Value |
|---|---|
| Electrical Conductivity | 0.003 S/m (diamond), 2-3×10^5 S/m (graphite) |
| Magnetic Susceptibility | Diamagnetic |
| Dielectric Constant | 5.7 (diamond), 10-15 (graphite) |
| Property | Description / Value |
|---|---|
| Atomic Number | 6 |
| Atomic Mass | 12.011 u |
| Neutron Cross Section | 0.0035 barns (for ¹²C) |
| Isotopes | ¹²C (98.93%), ¹³C (1.07%), ¹⁴C (trace, radioactive) |
| Radioactivity | ¹⁴C, half-life of 5,730 years |
Carbon dioxide (CO₂) is a colorless, odorless gas that is vital to life on Earth. It’s a chemical compound composed of one carbon atom covalently double bonded to two oxygen atoms. Here are some key aspects of carbon dioxide:
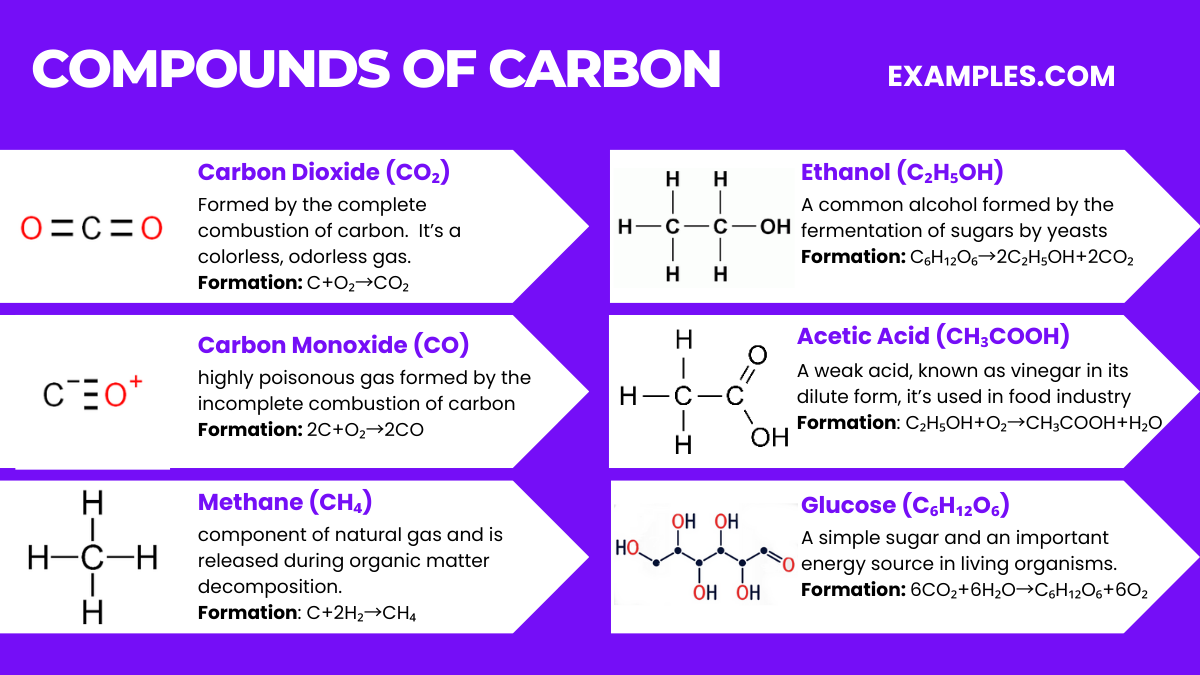
Carbon forms a vast array of compounds, given its ability to catenate and form covalent bonds with many elements. Here are some well-known compounds of carbon along with their chemical equations:
| Isotope | Atomic Number | Neutrons | Natural Abundance | Half-Life | Stability | Key Uses |
|---|---|---|---|---|---|---|
| Carbon-12 (¹²C) | 6 | 6 | 98.93% | Stable | Stable | Fundamental in life processes, standard for atomic weights |
| Carbon-13 (¹³C) | 6 | 7 | 1.07% | Stable | Stable | Used in NMR spectroscopy, environmental science, and geochemistry |
| Carbon-14 (¹⁴C) | 6 | 8 | Trace (<1%) | 5,730 years | Radioactive | Radiocarbon dating of archaeological, geological, and hydrogeological samples |
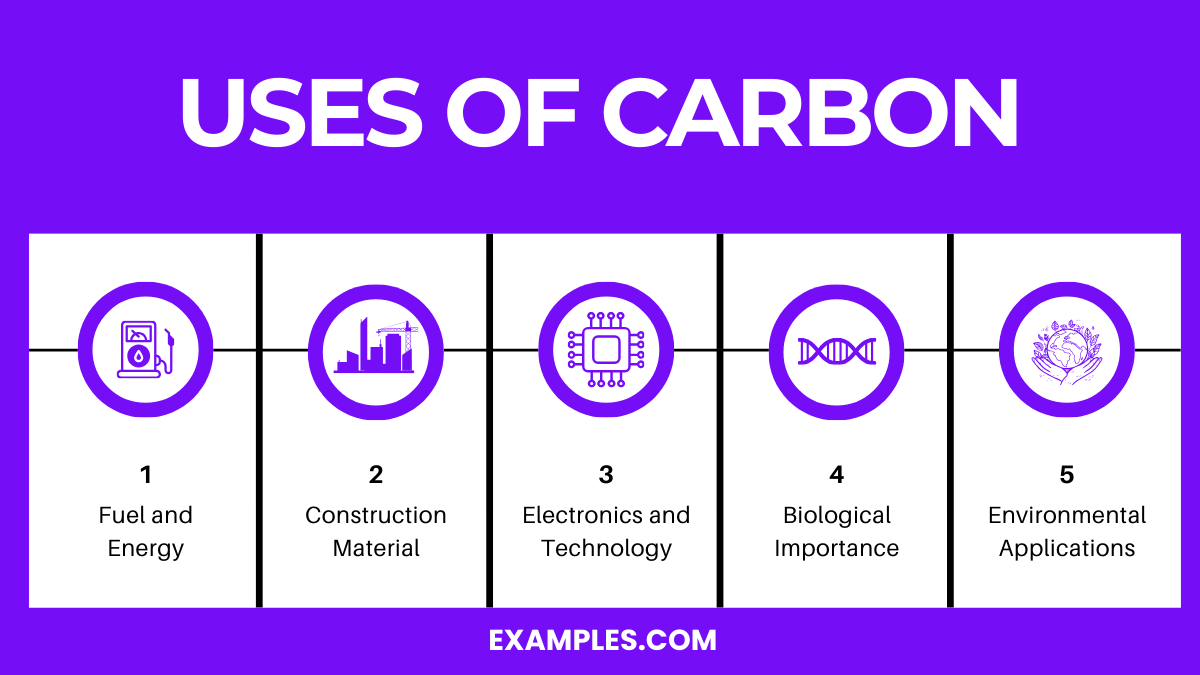
Carbon, a versatile element, is fundamental to various industrial, scientific, and biological applications. It forms a vast number of compounds, more than any other element, with almost ten million compounds described to date.
Carbon is essential for life, forming the basis of organic chemistry. It’s a key component in DNA, fuels, plastics, and Earth’s carbon cycle.
Carbon is obtained from natural sources like plants, fossil fuels (coal, oil, natural gas), and the atmosphere. It’s also produced industrially from carbonaceous materials.
Carbon forms diverse organic compounds and fuels, supports life as a biological building block, and plays a crucial role in climate regulation and ecosystems.
In real life, carbon is found in all living organisms, fuels like gasoline, materials like plastic and steel, and in the atmosphere as carbon dioxide.
While carbon is essential, excessive carbon emissions, primarily CO2, contribute to climate change, air pollution, and environmental degradation, posing significant health and ecological risks.
Understanding carbon is crucial due to its omnipresence in life and technology. From its role in organic chemistry to its impact on the environment, carbon is a double-edged sword. Embracing its benefits while mitigating its risks through informed usage and innovative solutions is key. This guide aims to enhance your understanding and application of carbon in various contexts.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the most stable form of carbon under standard conditions?
Graphite
Diamond
Fullerene
Carbon nanotubes
Which form of carbon is known for being the hardest naturally occurring substance?
Graphene
Diamond
Lonsdaleite
Charcoal
Carbon forms an essential compound with oxygen known as:
Carbon monoxide
Carbon dioxide
Carbon disulfide
Carbon tetrachloride
Which allotrope of carbon is a single layer of graphite?
Diamond
Carbon fiber
Graphene
Coal
What is the primary use of activated carbon?
Building material
Electrical conductor
Water filtration
Jewelry
Carbon-14 is used in which of the following techniques?
Alloy production
Radiocarbon dating
Electroplating
Photovoltaic cell manufacturing
What property of carbon nanotubes makes them particularly useful in materials science?
Flexibility
Electrical conductivity
Optical transparency
Solubility in water
Which form of carbon is used to manufacture the lead in pencils?
Graphite
Diamond
Charcoal
Carbon black
What unique property does fullerene exhibit due to its structure?
High thermal conductivity
Electrical insulation
Ability to act as a superconductor
Incombustibility
How does carbon contribute to the greenhouse effect?
Reflecting sunlight back into space
Absorbing heat in the atmosphere
Increasing oxygen levels
Forming carbon dioxide
Before you leave, take our quick quiz to enhance your learning!

