What type of element is Radon?
Metal
Nonmetal
Metalloid
Noble gas
Radon, a natural but elusive element, poses significant interest and challenges in both science and safety. This guide illuminates radon’s definition, characteristics, and its critical role in environmental health. Particularly important for educators, understanding radon is key in fostering awareness and safety in science classes. Dive into the world of radon, equipped with essential knowledge, vivid examples, and practical tips to enrich your teaching experience.
Radon is a colorless, odorless, radioactive gas, occurring naturally as a decay product of uranium. It’s the heaviest gas in the noble gases series and is considered a health hazard due to its radioactivity. Radon is invisible to the naked eye, yet its presence in homes and buildings can pose significant health risks, primarily lung cancer. Understanding its nature and impact is crucial in education to promote awareness and safety measures.
| Helium |
| Neon |
| Argon |
| Krypton |
| Xenon |
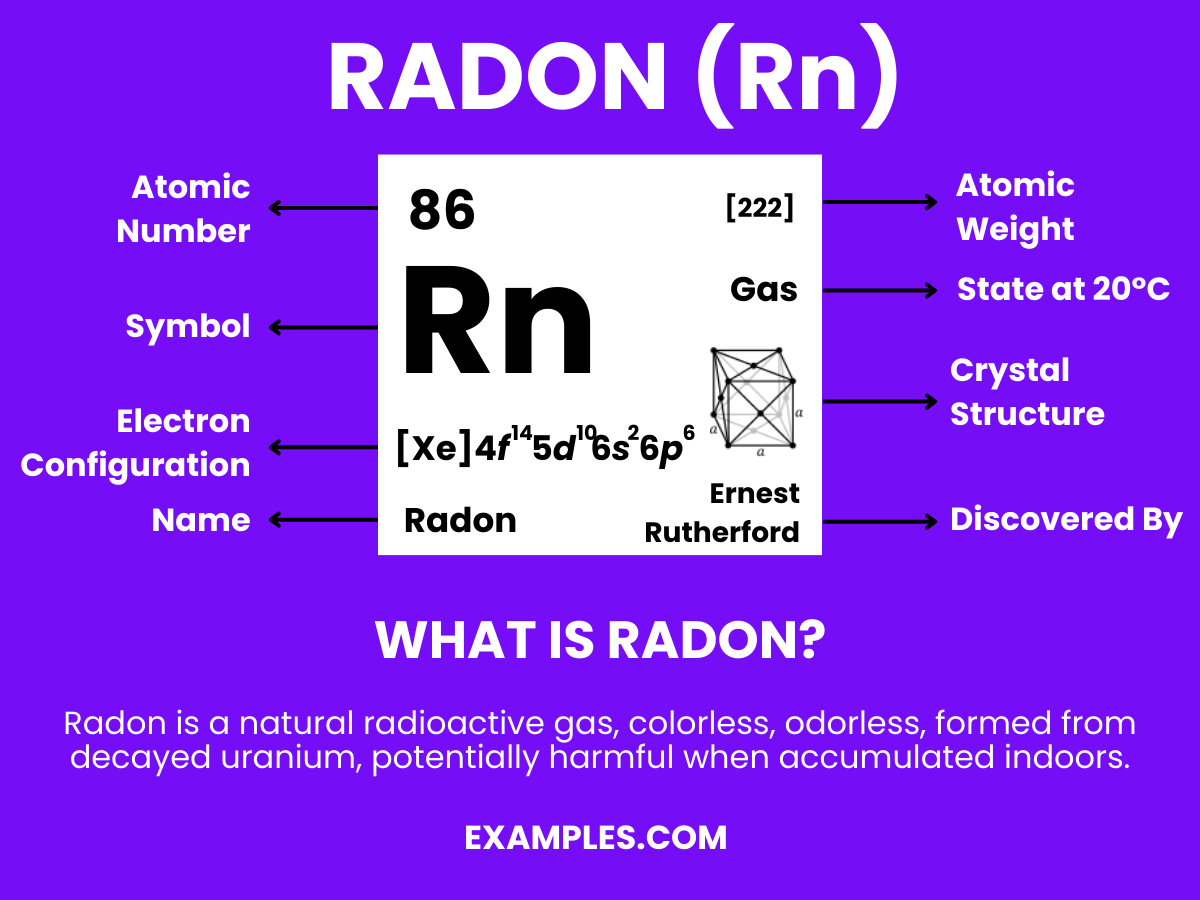
Formula: Rn
Composition: A single radon atom.
Bond Type: Typically non-reactive due to a complete valence shell.
Molecular Structure: Monatomic gas.
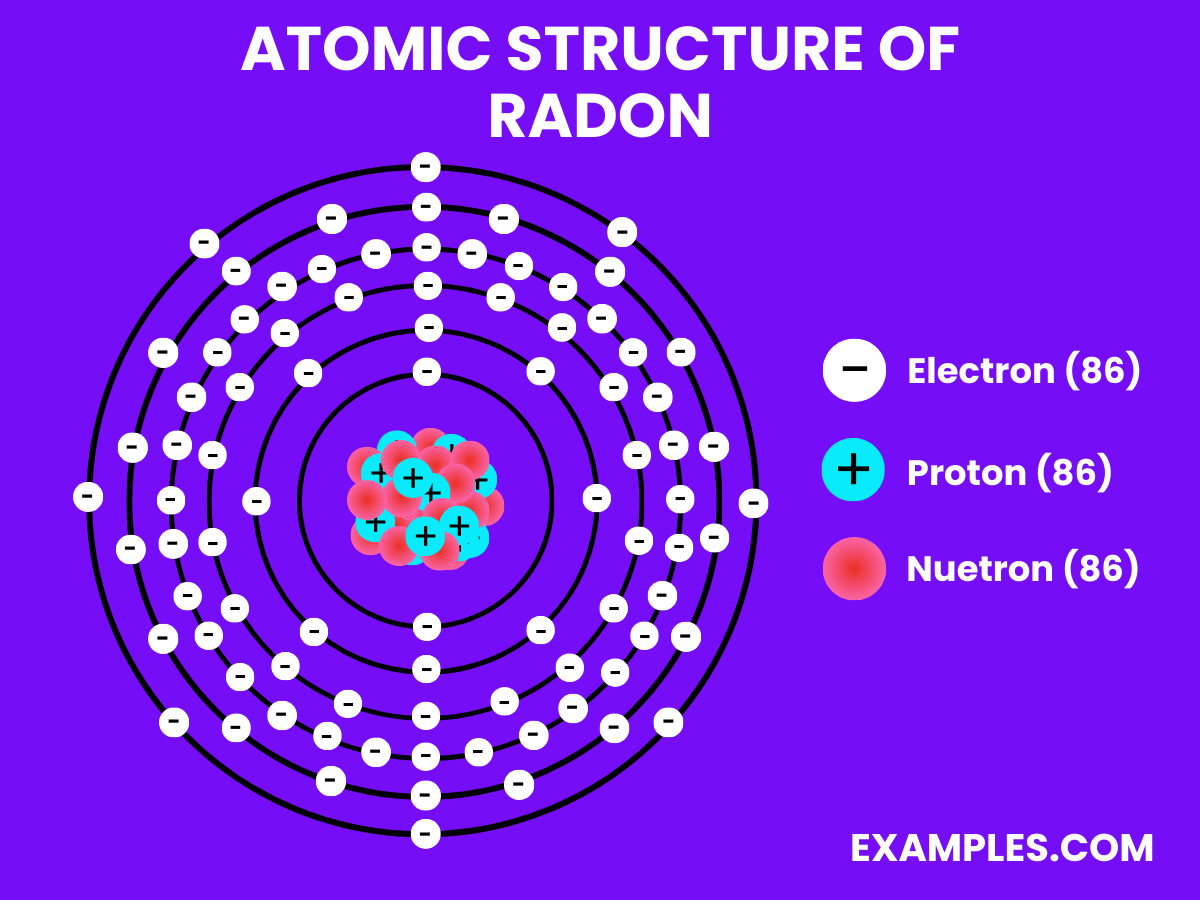
Electron Configuration: 86 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s² 6p⁶.
Significance: Limited use due to radioactivity; research interest in radiology.
Role in Chemistry: Inert, forms few compounds, mainly of academic interest.
| Physical Property | Description |
|---|---|
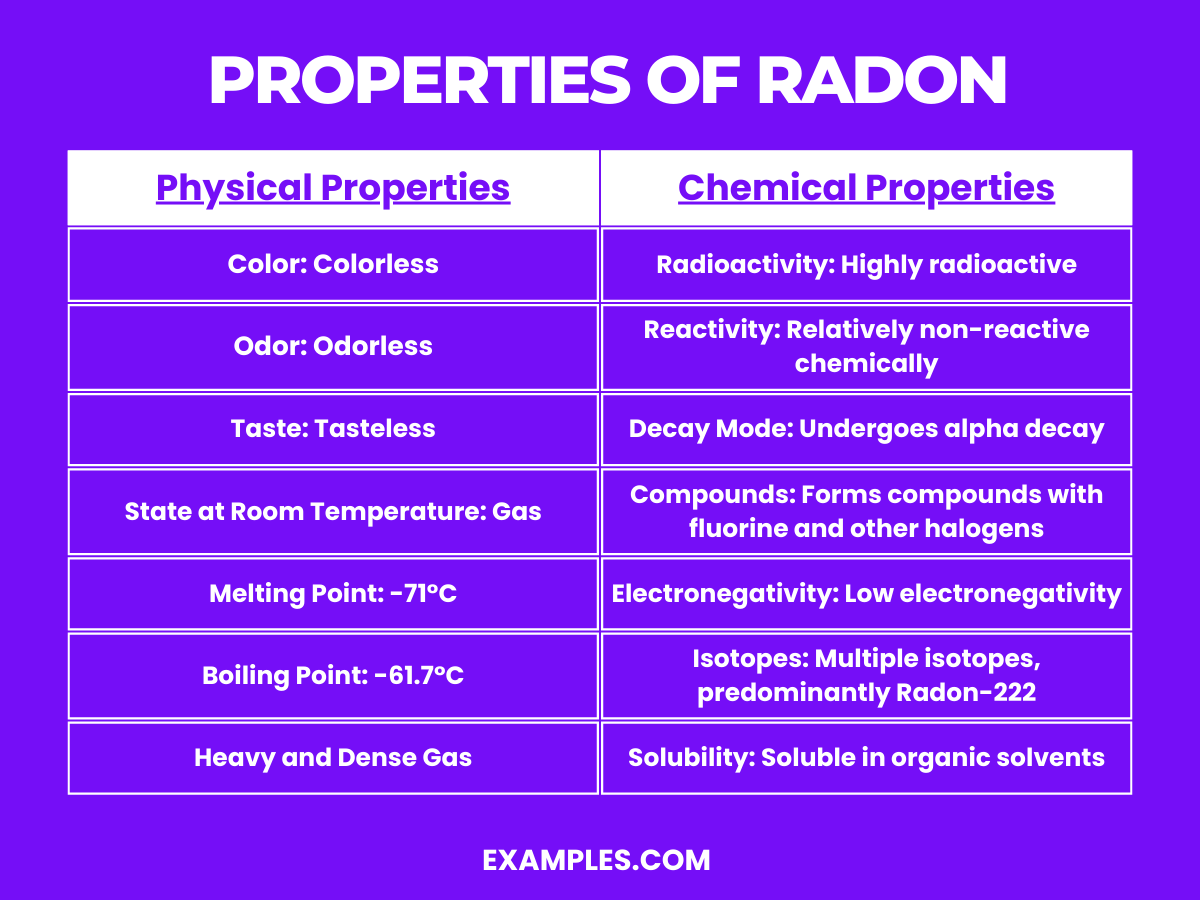
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| State at Room Temperature | Gas |
| Density | 9.73 kg/m³ at standard temperature and pressure (STP) |
| Melting Point | -71°C (-96°F) |
| Boiling Point | -61.7°C (-79°F) |
| Solubility in Water | Low; more soluble in organic solvents |
Radon is a unique element with several interesting chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | -71°C (-96°F) |
| Boiling Point | -61.7°C (-79.1°F) |
| Thermal Conductivity | 3.61 mW/(m·K) at 0°C |
| Specific Heat | 5.1 J/(mol·K) at 25°C (gas) |
| Heat of Vaporization | 18.10 kJ/mol at boiling point |
| Heat of Fusion | 3.247 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 9.73 kg/m³ at 0°C, 101.325 kPa (gas) |
| Approximately 4.4 g/cm³ at -61°C (liquid) | |
| Solubility in Water | 0.18 cm³/kg at 20°C |
| Color | Colorless (both in gas and liquid forms) |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Non-conductor (Radon is a noble gas and does not conduct electricity) |
| Property | Description / Value |
|---|---|
| Atomic Number | 86 |
| Atomic Mass | 222 u (most stable isotope, ^222Rn) |
| Neutron Cross Section | 0.7 barns (for ^222Rn) |
| Isotopes | Multiple isotopes, all radioactive |
| Radioactivity | Highly radioactive; ^222Rn (half-life: 3.8235 days), ^220Rn (half-life: 55.6 seconds), among others |
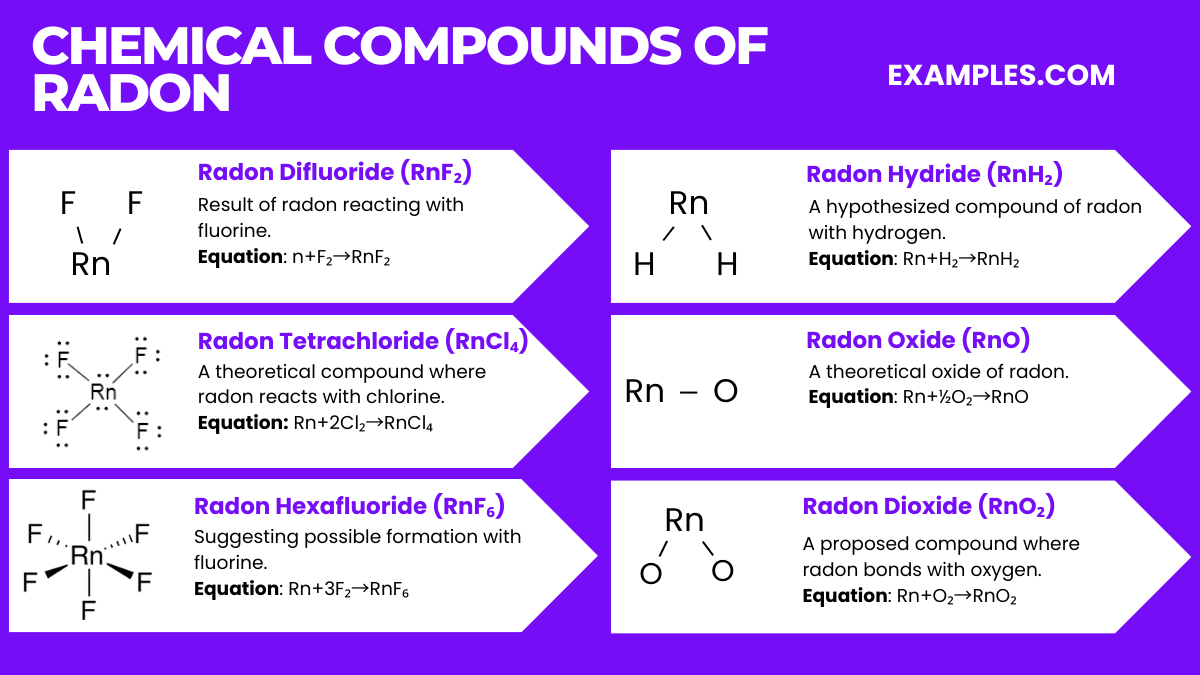
Radon, while generally inert, can form a few compounds under specific conditions. Here are six notable radon compounds along with their relevant chemical equations:
It’s important to note that most of these compounds are theoretical and have not been observed under normal laboratory conditions due to radon’s high radioactivity and short half-life.
Radon has several isotopes, each with unique characteristics. The table below describes some of the key isotopes of radon:
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| Rn-219 | 219 | 3.96 seconds | Alpha decay |
| Rn-220 | 220 | 55.6 seconds | Alpha decay |
| Rn-222 | 222 | 3.823 days | Alpha decay |
| Rn-224 | 224 | 1.8 hours | Alpha decay |
| Rn-226 | 226 | 30.9 minutes | Alpha decay |
| Rn-228 | 228 | 65.5 seconds | Alpha decay |
The isotopes of radon are all radioactive, with Radon-222 being the most stable and commonly encountered, especially in environmental and health-related contexts. Their radioactivity primarily involves alpha decay, contributing to radon’s significance in radiological health concerns.
Radon, despite being a radioactive noble gas, has some specialized uses:
Radon is not commercially produced in the same way as many other elements because of its radioactive nature and the health risks associated with its handling. Instead, it is usually obtained as a by-product of the decay of naturally occurring radioactive materials:
Radon is a radioactive gas that poses significant health risks, primarily when it accumulates in indoor environments:
Radon’s environmental effects are primarily related to its radioactivity and how it disperses in the environment:
Radon is used in cancer radiotherapy, geological earthquake prediction, hydrogeological studies, and scientific research due to its radioactivity.
Radon is highly toxic and radioactive, posing serious health risks such as lung cancer, especially when accumulated in indoor spaces.
Radon itself does not glow; however, its radioactive decay can cause other substances to fluoresce, typically not producing visible light.
Yes, radon is a noble gas, characterized by its radioactivity and lack of chemical reactivity under normal conditions.
Radon in homes typically presents no immediate symptoms; long-term exposure increases the risk of lung cancer, without specific radon-related symptoms.
Prolonged exposure to radon can lead to serious health issues, primarily lung cancer, due to its radioactive nature and alpha particle emission.
Radon, a naturally occurring radioactive noble gas, has critical applications in medicine and geology but poses significant health risks, especially lung cancer, upon indoor accumulation. Understanding its properties, ensuring regular home testing, and implementing mitigation strategies are essential for health and safety, highlighting the importance of awareness and responsible handling of radon in various environments.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What type of element is Radon?
Metal
Nonmetal
Metalloid
Noble gas
Radon is primarily produced by the decay of:
Uranium
Thorium
Radium
Plutonium
Which property of Radon makes it a health hazard?
Flammability
Radioactivity
Toxicity
Reactivity
Radon has the highest concentration in which type of environment?
Open fields
Urban areas
Indoor spaces
Coastal regions
The most common method to test for Radon levels in homes is:
Gas chromatography
Alpha-track detectors
Mass spectrometry
Liquid scintillation
Radon is chemically inert because it:
Has a complete valence shell
Is highly electronegative
Forms stable isotopes
Is a solid at room temperature
Exposure to Radon increases the risk of:
Skin cancer
Lung cancer
Liver cancer
Brain cancer
Radon's atomic number is:
86
88
89
92
To mitigate Radon in buildings, which technique is effective?
Air conditioning
Humidification
Ventilation
Filtration
The stable isotopes of Radon are:
Naturally occurring
Non-existent
Artificially produced
Highly abundant
Before you leave, take our quick quiz to enhance your learning!

