What is the atomic number of californium?
96
97
98
99
Unlock the mysteries of Californium, a powerhouse element in the realm of nuclear science and technology. This complete guide illuminates the fascinating aspects of Californium, from its synthesis to its groundbreaking applications in medicine, industry, and nuclear research. With examples illustrating its pivotal role, we explore how Californium’s unique properties are harnessed in cancer treatment, neutron radiography, and as a neutron source. Delve into the world of Californium, where advanced science meets practical innovation, offering a glimpse into the future of technological advancements and scientific discovery
| Actinium | Berkelium |
| Thorium | Fermium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
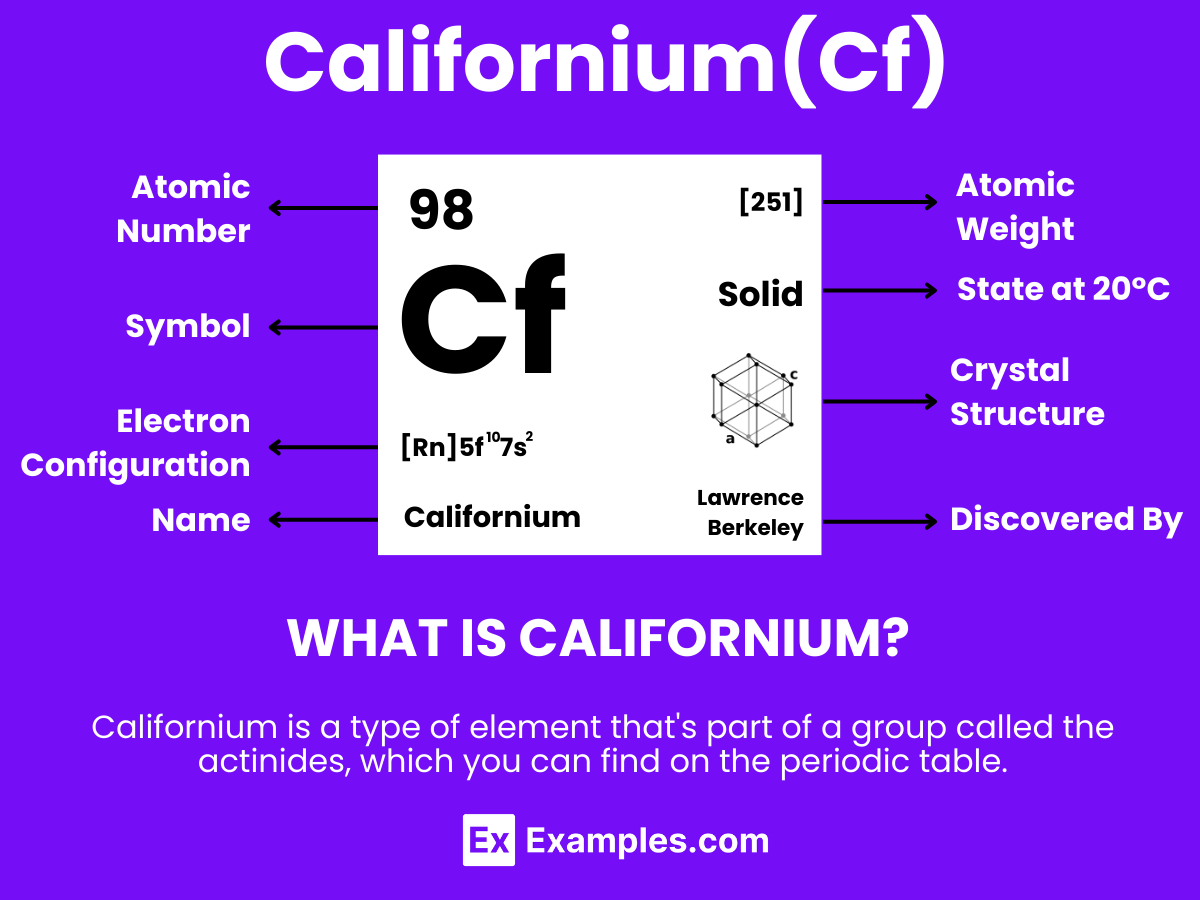
Californium, a synthetic radioactive element with the symbol Cf and atomic number 98, is part of the actinide series in the periodic table. Understanding its atomic structure offers insights into its chemical behavior, applications, and the broader context of nuclear science.
Californium is a synthetic, radioactive element with notable applications in science and industry. Here’s a concise overview of its key properties:
| Property | Description |
|---|---|
| Appearance | Silvery-white, lustrous metal |
| Atomic Number | 98 |
| Atomic Mass | 251 u (Californium-251 isotope) |
| Density (at room temperature) | 15.1 g/cm³ |
| Melting Point | 900 °C (1652 °F) |
| Boiling Point | 1743 °C (3170 °F) |
| State at Room Temperature | Solid |
| Crystal Structure | Hexagonal |
| Thermal Conductivity | 10 W/(m·K) |
| Electrical Conductivity | Poor conductor of electricity |
| Radioactivity | Highly radioactive, emitting alpha particles |
Californium exhibits fascinating chemical properties due to its position in the actinide series. Its behavior is similar to other early actinides, with a few distinct characteristics:
The thermodynamic properties of Californium provide insight into its behavior under various conditions of temperature and pressure. Below is a table summarizing these key properties:
| Property | Value |
|---|---|
| Melting Point | 900 °C (1652 °F) |
| Boiling Point | 1470 °C (2678 °F) |
| Density at Room Temperature | ~15.1 g/cm³ |
| Heat of Fusion | Estimated 8-10 kJ/mol |
| Heat of Vaporization | Estimated 420 kJ/mol |
| Specific Heat Capacity | No specific data available |
| Thermal Conductivity | No specific data available |
The material properties of Californium highlight its physical characteristics and how it interacts with its environment:
| Property | Value |
|---|---|
| State at Room Temperature | Solid |
| Color | Silvery |
| Phase at Room Temperature | Solid |
| Hardness | No specific data available |
| Elastic Modulus | No specific data available |
| Poisson’s Ratio | No specific data available |
| Thermal Expansion Coefficient | No specific data available |
The electromagnetic properties of Californium relate to its behavior in the presence of electric and magnetic fields:
| Property | Value |
|---|---|
| Electrical Conductivity | No specific data available |
| Magnetic Susceptibility | Paramagnetic at room temperature |
| Electrical Resistivity | No specific data available |
The nuclear properties of Californium are critical for understanding its radioactive behavior and applications:
| Property | Value |
|---|---|
| Isotopes | Primarily Cf-249, Cf-250, Cf-251, and Cf-252 |
| Half-life of Cf-252 | 2.645 years |
| Alpha decay energies | Varies by isotope, generally between 5-6 MeV |
| Spontaneous Fission (Cf-252) | 3.09×10^12 fissions/mg/hour |
| Neutron Emission (Cf-252) | Significant source of neutrons |
Californium, a synthetic and radioactive element, is produced through neutron bombardment of lighter elements like curium in nuclear reactors or particle accelerators. The process involves several steps:
A brown solid used for research on californium’s chemical properties. Formation
equation: 2Cf+3O₂→Cf2O₃
A green solid that helps study californium’s behavior in different states.
Synthesis reaction: 3Cf+23Cl₂→CfCl₃
A white solid offering insights into californium’s reactivity with oxygen and chlorine.
Produced by: Cf2O₃+2HCl→2CfOCl+H₂O
A black solid that contributes to understanding the oxidation states of californium.
Formation process: 2Cf+O₂→CfO2
A volatile compound used in studying californium’s fluorine chemistry. Synthesized through:
4Cf+2F₂→CfF₄
A yellow solid, aiding in the exploration of californium’s ionic compounds.
Preparation equation: 3Cf+23I₂→CfI₃
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Cf-248 | 333.5 days | Alpha decay |
| Cf-249 | 351 years | Alpha decay |
| Cf-250 | 13.08 years | Alpha decay, spontaneous fission |
| Cf-251 | 898 years | Alpha decay |
| Cf-252 | 2.645 years | Alpha decay, spontaneous fission |
| Cf-253 | 17.81 days | Alpha decay |
| Cf-254 | 60.5 days | Spontaneous fission |
The production of Californium involves sophisticated nuclear reactions, primarily occurring within high-flux nuclear reactors. The process starts with the bombardment of curium isotopes (typically Curium-242 or Curium-244) with neutrons, leading to a series of neutron captures and beta decays, ultimately resulting in the formation of Californium isotopes. The most common method includes:
This complex process yields relatively small amounts of Californium, making it one of the most expensive elements to produce, with Californium-252 being one of the most commonly produced isotopes due to its high neutron emission rate.
Californium’s unique properties, especially its high neutron emission rate, make it invaluable in a variety of applications:
Californium is a highly radioactive and synthetic element, renowned for its use in neutron sources and scientific research. Its complex thermodynamic, material, electromagnetic, and nuclear properties highlight its uniqueness. Despite the challenges in handling and scarcity, Californium’s contributions to science and technology underscore its significance, offering potential for future applications in various fields.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of californium?
96
97
98
99
In which year was californium first synthesized?
1945
1950
1955
1960
What is the symbol for californium?
Cf
Cl
Ca
Cm
Californium belongs to which series in the periodic table?
Lanthanides
Actinides
Transition metals
Halogens
What is a primary use of californium-252?
Cancer treatment
Radiography
Neutron activation analysis
Smoke detectors
What is the half-life of californium-252?
2.6 years
8.5 years
15.1 years
21.5 years
Californium was named after which location?
California
University of California
California Institute of Technology
Both a and b
Californium is primarily produced in which type of facility?
Nuclear reactors
Particle accelerators
Chemical plants
Solar panels
Which scientist was NOT involved in the discovery of californium?
Glenn T. Seaborg
Stanley G. Thompson
Albert Ghiorso
Enrico Fermi
What is the oxidation state of californium in most of its compounds?
+2
+3
+4
+5
Before you leave, take our quick quiz to enhance your learning!

