What is the atomic number of Silver?
46
47
48
49
Dive into the lustrous world of Silver, a precious metal that captivates with its versatility and brilliance. This comprehensive guide illuminates Silver’s rich history, its pivotal role in various industries, and the myriad of uses it offers, from jewelry making to technological applications. We’ll explore the elemental properties that make Silver so valuable, delve into its fascinating compounds, and showcase real-world examples. Whether for investment, industry, or innovation, Silver’s allure remains unmatched, promising endless possibilities.
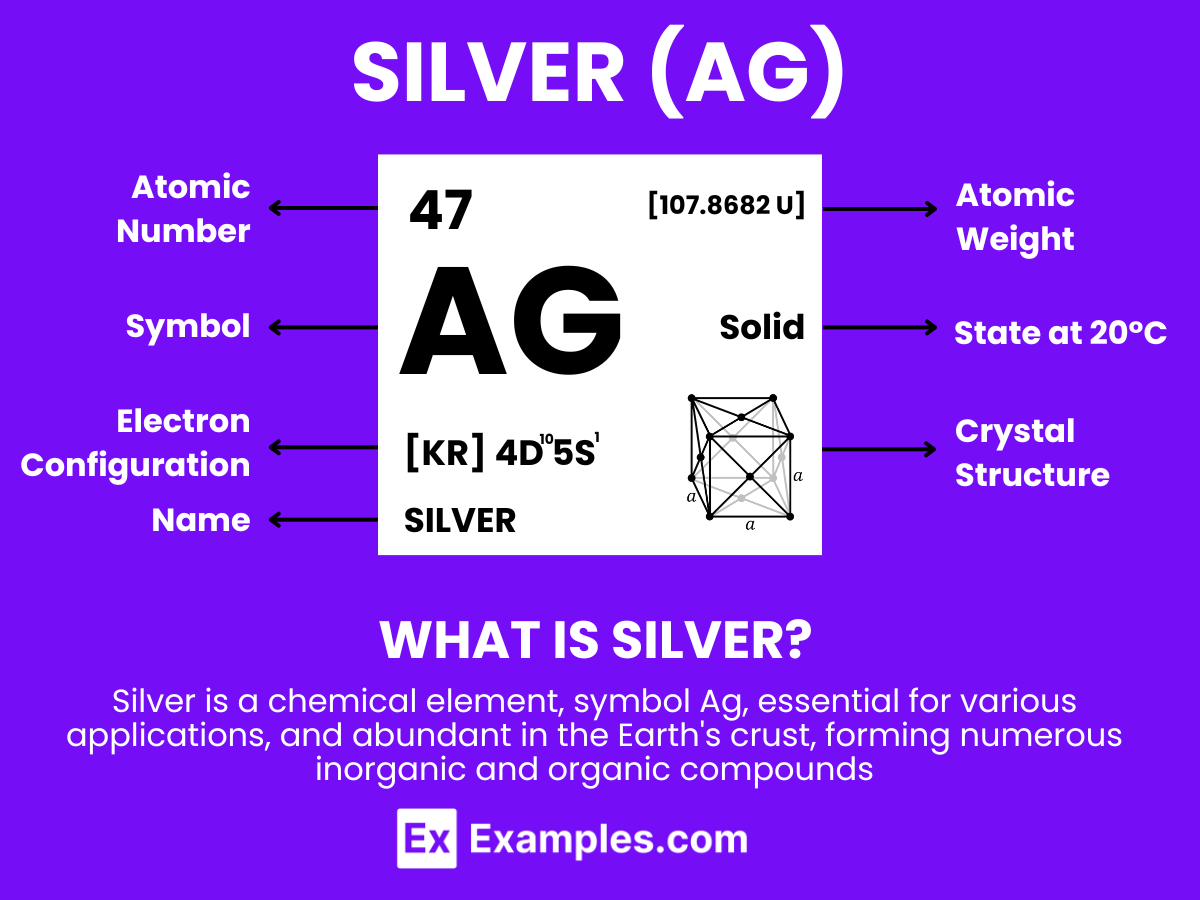
Silver is a chemical element with the symbol Ag and atomic number 47. It is a lustrous, white, soft, very ductile, and malleable metal. Known for its highest electrical and thermal conductivity of any metal, Silver is extensively used in a wide array of applications. Its inherent properties, such as reflectivity and resistance to corrosion, make it highly valuable not only for industrial purposes but also in jewelry, silverware, and photography. In industries like electronics, medicine, and energy, Silver plays a pivotal role due to its conductive and antimicrobial characteristics. .
Silver, symbol Ag (from the Latin ‘argentum’), with atomic number 47, is a precious metal known for its lustrious appearance and exceptional electrical conductivity. At an atomic level, silver exhibits a unique structure characterized by the following aspects:
The atomic structure of silver, with its filled d orbitals and single s orbital electron, plays a crucial role in its physical and chemical properties, making it invaluable in industries ranging from jewelry and coinage to electronics and photography.
| Property | Value |
|---|---|
| Appearance | Lustrous, white, metallic |
| Atomic Number | 47 |
| Atomic Weight | 107.8682 u |
| Density | 10.49 g/cm³ at 20 °C |
| Melting Point | 961.78 °C |
| Boiling Point | 2162 °C |
| Electrical Conductivity | Highest of all metals |
| Thermal Conductivity | 429 W/(m·K) at 25 °C |
| State at Room Temperature | Solid |
| Crystal Structure | Face-Centered Cubic (FCC) |
| Malleability | High, can be beaten into extremely thin sheets |
| Ductility | High, can be drawn into very thin wires |
| Reflectivity | Very high, reflects over 95% of the visible light spectrum |
Silver, with its symbol Ag, is a noble metal known for its low reactivity, but it still participates in several chemical reactions under specific conditions. Here are key chemical properties of silver:
| Property | Value |
|---|---|
| Melting Point | 961.78 °C |
| Boiling Point | 2162 °C |
| Heat of Fusion | 11.28 kJ/mol |
| Heat of Vaporization | 254 kJ/mol |
| Specific Heat Capacity | 25.35 J/(mol·K) at 25 °C |
| Property | Value |
|---|---|
| Density | 10.49 g/cm³ at 20 °C |
| Malleability | High, can be beaten into extremely thin sheets |
| Ductility | High, can be drawn into very thin wires |
| Tensile Strength | 170–200 MPa |
| Hardness (Mohs) | 2.5 – 3 |
| Thermal Expansion Coefficient | 18.9 µm/(m·K) at 25 °C |
| Property | Value |
|---|---|
| Electrical Conductivity | Highest of all metals, 63.01 x 10^6 S/m |
| Thermal Conductivity | 429 W/(m·K) at 25 °C |
| Magnetic Susceptibility | -19.5 x 10^-6 cm^3/mol (diamagnetic) |
| Property | Value |
|---|---|
| Natural Isotopes | Ag-107 (51.839%), Ag-109 (48.161%) |
| Radioisotopes | Ag-105, Ag-111, among others |
| Neutron Cross Section | 63.3 barns (for Ag-107) |
| Isotopic Abundance | Ag-107: 51.839%, Ag-109: 48.161% |
The preparation of silver involves extracting the metal from its ores and refining it to achieve high purity. Silver is commonly found in nature in its native form and as a part of various minerals such as argentite (silver sulfide) and horn silver (silver chloride).
| Isotope | Mass Number | Natural Abundance (%) | Half-life | Notes |
|---|---|---|---|---|
| Ag-107 | 107 | 51.839 | Stable | – |
| Ag-109 | 109 | 48.161 | Stable | – |
| Ag-105 | 105 | – | 41.2 days | Radioactive, used in research |
| Ag-111 | 111 | – | 7.45 days | Used in nuclear medicine |
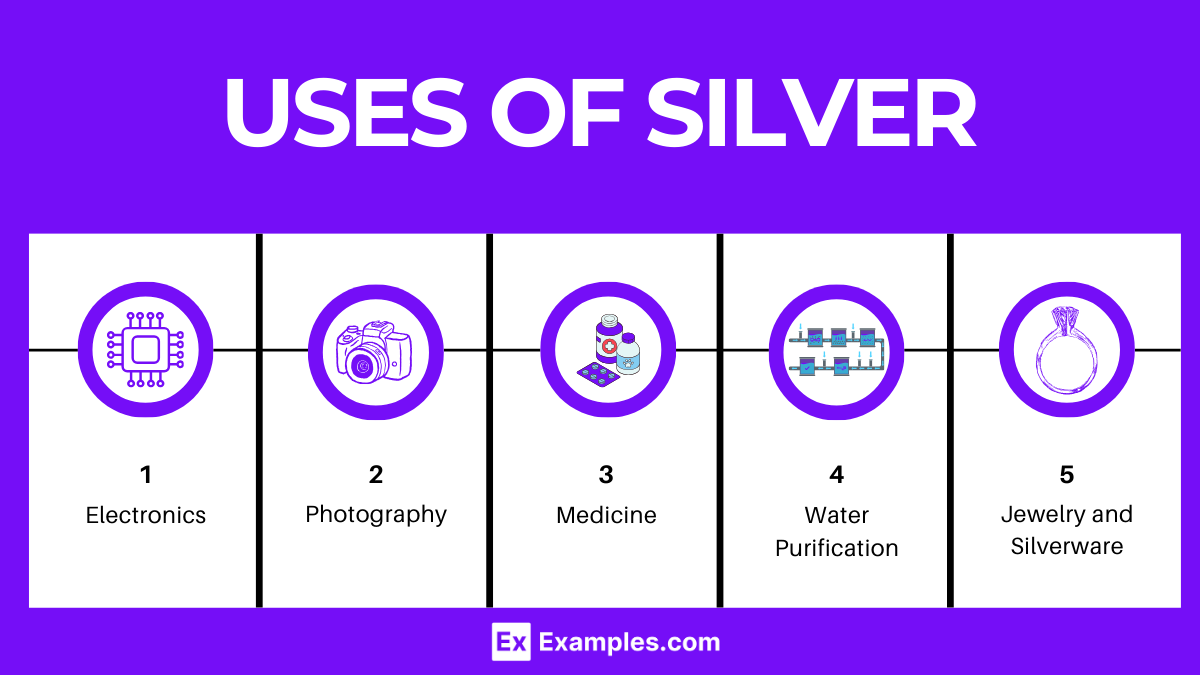
Silver’s unique properties, including its thermal and electrical conductivity, reflectivity, and antimicrobial nature, contribute to its wide-ranging applications:
Silver production involves several steps, from mining the ore to refining it into pure silver. The process can be summarized as follows:
Silver’s unique properties have made it indispensable in various fields:
silver, with its unmatched electrical conductivity, thermal properties, and antimicrobial qualities, remains a cornerstone in numerous industries. From electronics and medicine to jewelry and sustainable energy solutions, silver’s versatility and value are unparalleled. This table of silver not only highlights its importance but also underlines its indispensability in advancing modern technology and improving our daily lives.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Silver?
46
47
48
49
What is the chemical symbol for Silver?
Ag
Au
Si
Sb
Silver is primarily extracted from which ore?
Hematite
Bauxite
Galena
Argentite
Which property of Silver makes it ideal for use in electrical contacts?
High melting point
High thermal conductivity
High electrical conductivity
High density
What is the molar mass of Silver?
107.87 g/mol
108.87 g/mol
109.87 g/mol
110.87 g/mol
Silver nitrate is commonly used in which field?
Agriculture
Photography
Medicine
Construction
Which alloy contains Silver and is used in jewelry?
Brass
Bronze
Sterling silver
Pewter
What is the primary use of Silver in the medical field?
Surgical instruments
Antimicrobial agents
Diagnostic equipment
Implants
Which property of Silver makes it useful in mirrors?
High reflectivity
Low density
High hardness
Low melting point
What is the melting point of Silver?
800.0°C
1200.0°C
500.0°C
961.8°C
Before you leave, take our quick quiz to enhance your learning!

