What is the atomic number of Magnesium?
10
12
14
16

Magnesium plays a crucial role in various aspects of our lives and is a topic of great interest in both science and health education. This guide is designed to provide a comprehensive understanding of Magnesium, tailored for educators and students. We’ll explore its definition, importance in everyday life, and practical tips for incorporating this knowledge into the classroom. By understanding Magnesium’s role, teachers can effectively convey its significance to students, fostering an engaging and informative learning environment.
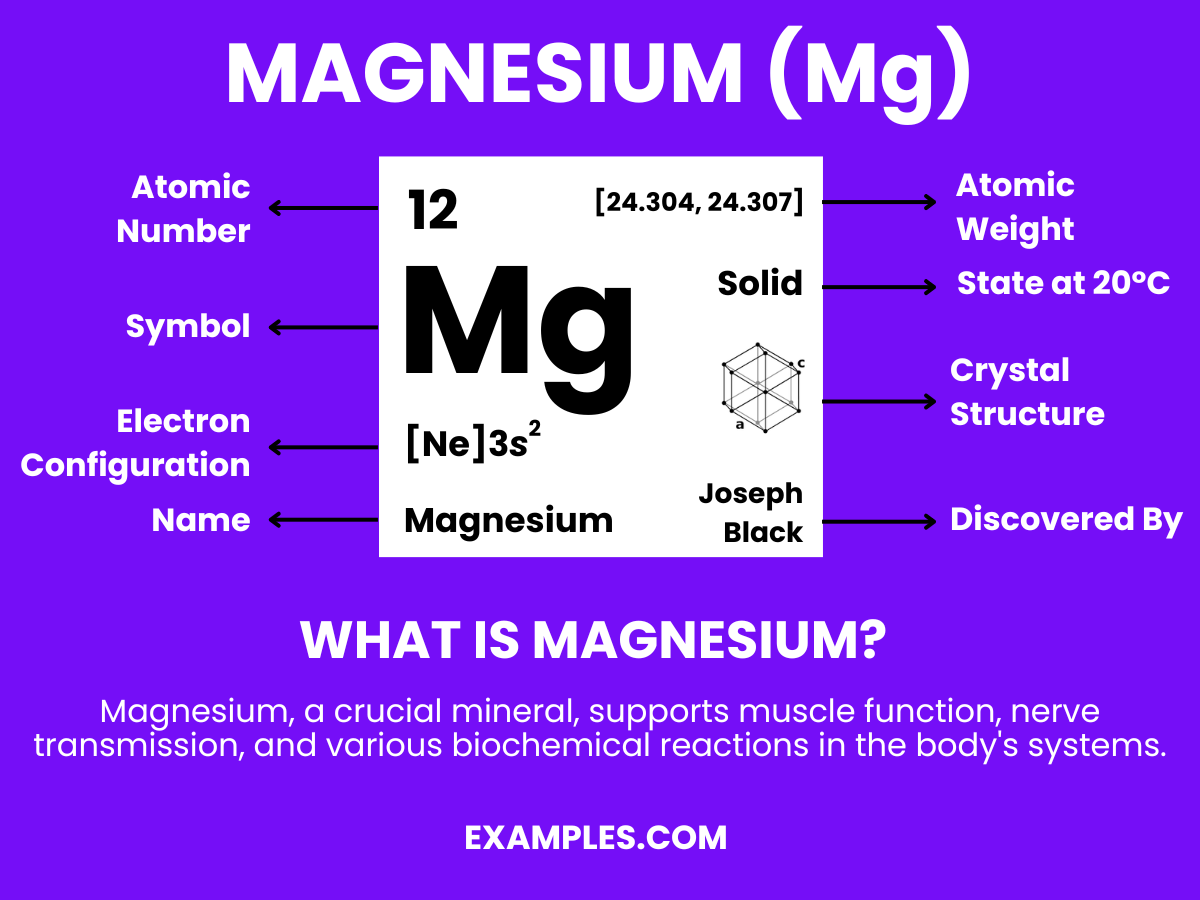
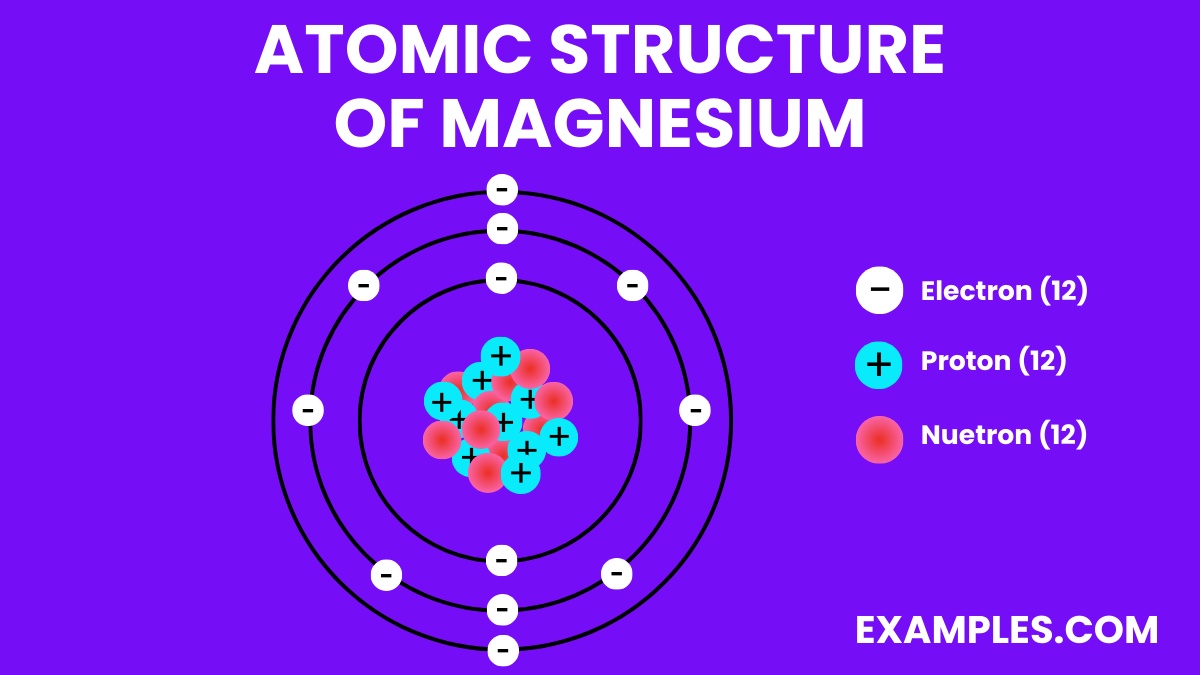
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. In simple terms, Magnesium is a vital mineral that is necessary for many bodily functions, including muscle and nerve function, blood glucose control, and bone health. It’s also involved in over 300 biochemical reactions in the body. For educators, understanding and teaching about Magnesium can enhance students’ knowledge in chemistry, biology, and health sciences.
| Beryllium(Be) |
| Calcium(Ca) |
| Strontium(Sr) |
| Barium(Ba) |
| Radium(Ra) |
| Property | Description |
|---|---|
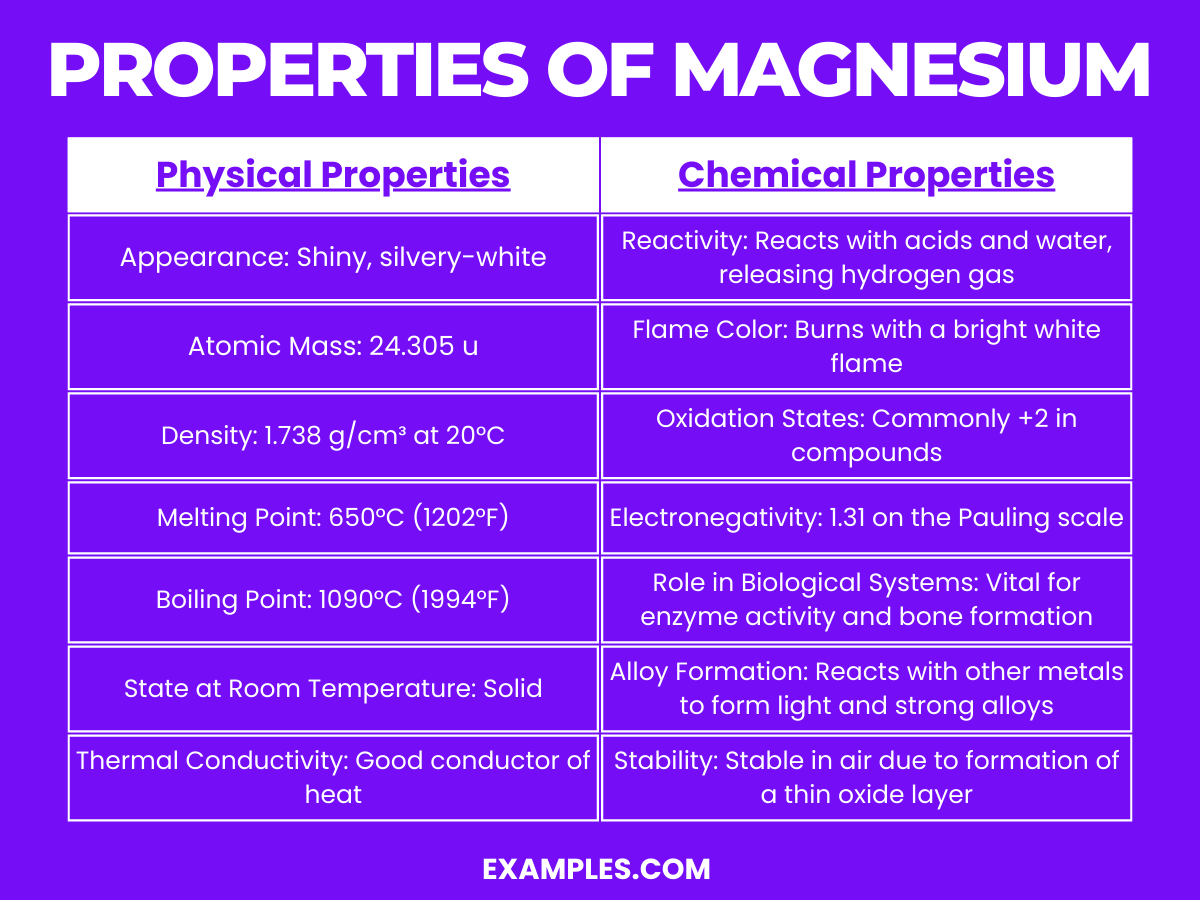
| Appearance | Silvery-white metal |
| Atomic Number | 12 |
| Atomic Mass | Approximately 24.305 u |
| Density | About 1.738 g/cm³ at room temperature |
| Melting Point | 650°C (1202°F) |
| Boiling Point | 1091°C (1994°F) |
| State at Room Temp | Solid |
| Electrical Conductivity | Good conductor of electricity |
| Property | Description / Value |
|---|---|
| Melting Point | 650°C (1202°F) |
| Boiling Point | 1091°C (1994°F) |
| Thermal Conductivity | 156 W/(m·K) |
| Specific Heat | 1.025 J/(g·K) at 25°C |
| Heat of Vaporization | 128 kJ/mol at boiling point |
| Heat of Fusion | 8.48 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 1.738 g/cm³ at 20°C |
| Young’s Modulus | 45 GPa |
| Tensile Strength | 200 to 280 MPa |
| Mohs Hardness | 2-2.5 |
| Elastic Modulus | 45 GPa |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 22.7 MS/m at 20°C |
| Property | Description / Value |
|---|---|
| Atomic Number | 12 |
| Atomic Mass | 24.305 u |
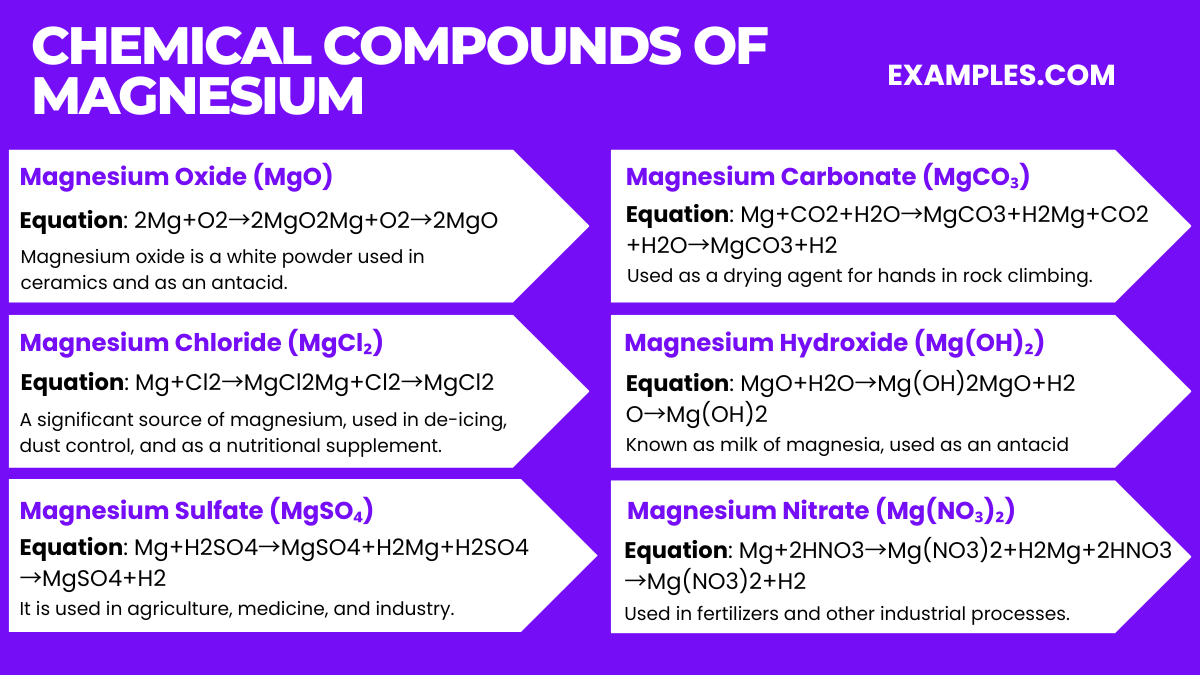
| Neutron Cross Section | 0.063 barns (for ^24Mg) |
| Isotopes | ^24Mg (79%), ^25Mg (10%), ^26Mg (11%) |
| Radioactivity | Magnesium has no stable radi |
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Mg-24 | 24 | 78.99 | Stable | Most abundant isotope, used in isotope analysis. |
| Mg-25 | 25 | 10.00 | Stable | Used in studies of magnesium metabolism and environmental tracing. |
| Mg-26 | 26 | 11.01 | Stable | Involved in cosmogenic studies, as it is produced by the decay of Al-26. |
| Mg-28 | 28 | – | 21 hours | Radioactive, produced in particle accelerators. |
| Mg-26m | 26 | – | 9.5 minutes (meta-state) | Has a higher energy state than normal Mg-26. |
| Mg-27 | 27 | – | 9.45 minutes | Radioactive, used in scientific research. |
Magnesium is produced commercially through several methods, the most common being the electrolysis of magnesium chloride and the Pidgeon process.
Magnesium is also obtained through the mining of minerals like magnesite and dolomite. However, these methods are less common compared to the electrolysis of magnesium chloride and the Pidgeon process.
Magnesium plays a vital role in human health. It is essential for the proper functioning of numerous biochemical processes and physiological functions.
Deficiency in magnesium can lead to health issues like osteoporosis, heart disease, muscle weakness, and mental disorders. On the other hand, excessive intake, though rare, can cause problems like diarrhea, nausea, and abdominal cramping.
Magnesium, being a naturally occurring element, is generally not harmful to the environment. However, its extraction and processing can have environmental impacts.
Magnesium aids in nerve function, muscle movement, energy production, and supports a healthy immune system. Essential for over 300 biochemical reactions in the body.
Yes, daily magnesium intake is safe and beneficial for most people, aiding in maintaining muscle and nerve function, heart rhythm, and bone strength.
Symptoms include muscle cramps, fatigue, weakness, nausea, loss of appetite, and, in severe cases, numbness and heart rhythm changes.
Almonds, spinach, cashews, peanuts, and soymilk are among the top sources of magnesium, offering a healthy, natural intake of this vital mineral.
Magnesium is widely used in manufacturing light alloys, as an agent in producing other metals, in medications, and as an essential dietary mineral.
Magnesium is a chemical element with the symbol Mg and atomic number 12, classified as an alkaline earth metal in the periodic table.
Magnesium is abundantly found in the Earth’s crust, sea water, and in minerals like dolomite, magnesite, and epsomite, making it widely available for various uses.
Magnesium is a versatile and vital element, essential for numerous bodily functions and a wide range of industrial applications. Understanding its properties, sources, and uses enriches our knowledge and highlights its importance in daily life. Whether in nutrition, manufacturing, or chemistry, magnesium’s role is indispensable, underscoring the need to appreciate and utilize this remarkable element wisely.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Magnesium?
10
12
14
16
Which property of magnesium makes it highly useful in the manufacturing of lightweight materials?
Reactivity with water
High melting point
Low density
High thermal conductivity
Magnesium is an essential element for:
Only animal cells
Only plant cells
Both plant and animal cells
Neither plant nor animal cells
The reaction of magnesium with water at room temperature produces:
Magnesium oxide
Magnesium hydroxide
Magnesium chloride
No reaction
Which vitamin is magnesium associated with to improve its absorption in the human body?
Vitamin C
Vitamin D
Vitamin B12
Vitamin A
Magnesium alloys are commonly used in:
Construction of buildings
Manufacture of fireworks
Production of batteries
Jewelry making
The primary source of magnesium for industrial use is:
Seawater
Limestone
Dolomite
River water
Which of the following is NOT a common symptom of magnesium deficiency?
Muscle cramps
High blood pressure
Increased energy
Irregular heartbeat
Magnesium is important in the medical field for treating:
Hypertension
Skin infections
Bone fractures
Hair loss
The flame test color for magnesium is:
Red
Blue
Green
White
Before you leave, take our quick quiz to enhance your learning!

