Sodium Bicarbonate (NaHCO3) – Definition, Structure, Preparation, Uses, Benefits, Side Effects
Sodium bicarbonate, commonly known as baking soda, is an ionic compound with the chemical formula NaHCO₃. It plays a versatile role in various fields, from cooking, where it acts as a leavening agent, to medicine, where it’s used as an antacid to relieve heartburn and acid indigestion. The compound is made up of sodium (Na) ions and bicarbonate (HCO₃) ions, showcasing its ionic nature. In chemistry, sodium bicarbonate is appreciated for its ability to react with acids and bases, making it a valuable substance in neutralizing reactions and in experiments that require precise pH adjustments. Its widespread use and chemical properties make it a fascinating subject of study in the world of chemistry.
What is Sodium Bicarbonate?
Sodium bicarbonate is a versatile compound with a wide range of applications, extending beyond its popular use in baking to act as a leavening agent that causes dough to rise. Its chemical properties make it effective for neutralizing acids, leading to its employment in heartburn remedies and as a key ingredient in antacid medications. Its ability to release carbon dioxide when reacting with an acid is exploited in cooking and baking, while its mild abrasive qualities and odor neutralizing capabilities are valued in household cleaning and personal care products. Additionally, in medical settings, sodium bicarbonate is used to correct acid-base imbalances in the blood, showcasing its critical role in both everyday life and health care practices.
Structure of Sodium Bicarbonate
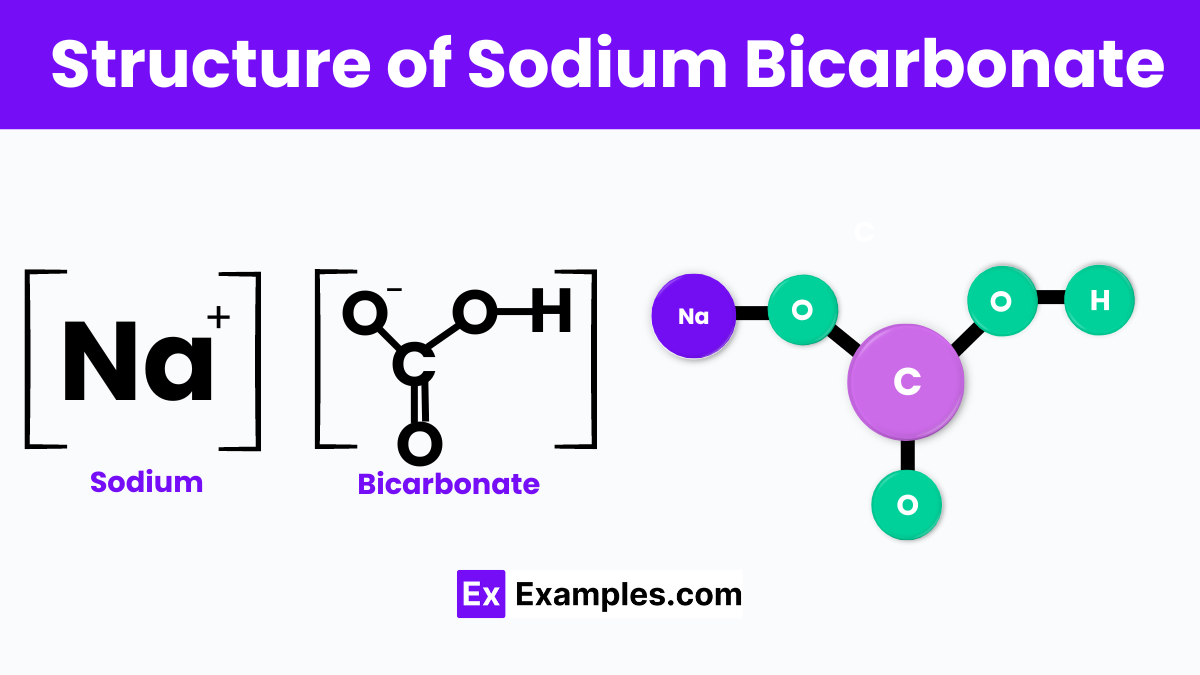
Sodium bicarbonate is a compound with the chemical formula NaHCO₃, comprising sodium ions (Na⁺) and bicarbonate ions (HCO₃⁻). The bicarbonate ion is the centerpiece of its structure, featuring a carbon atom double-bonded to an oxygen atom, single-bonded to a hydroxyl group (OH), and single-bonded to another oxygen atom carrying a negative charge. This configuration grants the compound its polar nature and allows it to play a pivotal role in various chemical reactions, especially in water where it readily dissolves, dissociating into sodium and bicarbonate ions.
In its crystalline form, sodium bicarbonate is part of the monoclinic crystal system, characterized by a stable lattice where each sodium ion is surrounded by six bicarbonate ions in a slightly distorted octahedral coordination. This structure is essential for its solubility in water and its reactivity with acids, which is exploited in baking, medical treatments, and cleaning applications. When sodium bicarbonate reacts with acids, it produces carbon dioxide, water, and a salt, a reaction that is fundamental to its use as a leavening agent in baking and its effectiveness in neutralizing acids in various settings.
Preparation of Sodium Bicarbonate
Sodium bicarbonate, commonly known as baking soda, is prepared through a process known as the Solvay process. This method involves the reaction of sodium chloride (salt) with ammonia and carbon dioxide in water. The process begins with the introduction of these reactants in a concentrated solution, leading to the formation of sodium bicarbonate and ammonium chloride. The key chemical reaction can be summarized as follows:
This equation highlights the transformation of the initial reactants into sodium bicarbonate and ammonium chloride.
In the Solvay process, the formed sodium bicarbonate is insoluble and precipitates out of the solution, allowing for its collection and purification. It is then heated to release carbon dioxide and water, converting it into sodium carbonate, also known as washing soda. However, for the production of sodium bicarbonate, the conditions are maintained to favor its formation and recovery. The process’s efficiency and sustainability come from its ability to recycle ammonia, using it repeatedly in the reaction cycle. This production method is widely used due to its cost-effectiveness and minimal environmental impact, making sodium bicarbonate readily available for various applications ranging from baking to cleaning.
Physical Properties of Sodium Bicarbonate
Physical Properties of Sodium Bicarbonate
| Property | Value/Description |
|---|---|
| Appearance | White crystalline solid |
| Odor | Odorless |
| Molecular Formula | NaHCO₃ |
| Molecular Weight | 84.006 g/mol |
| Density | 2.20 g/cm³ (solid) |
| Melting Point | Decomposes at 50°C (122°F) into sodium carbonate, water, and carbon dioxide |
| Solubility in Water | 9 g/100 mL (at 20°C) |
| pH | Alkaline (8.3 when dissolved in water) |
| Thermal Stability | Decomposes upon heating |
| Crystal Structure | Monoclinic |
Chemical Properties of Sodium Bicarbonate
Reacts with Acids
Sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (CO₂) gas, useful in baking and cleaning.
Equation: NaHCO₃+CH₃COOH→CH3₃COONa+H₂O+CO₂(g)
Thermal Decomposition
Upon heating, it decomposes into sodium carbonate (Na₂CO₃), water (H₂O), and CO₂, a reaction employed in fire extinguishers.
Equation: 2NaHCO₃(s)→Na₂CO₃(s)+H₂O(l)+CO₂(g)
Moderate Solubility in Water
Its solubility increases with temperature, and it helps maintain equilibrium between NaHCO₃, Na₂CO₃, CO₂, and H₂O in solution.
pH Regulation
Acts as a buffer, moderating pH changes in pools and the body by reacting with both acids and bases.
Formation of Complexes
Forms complexes with metal ions in solution, aiding in water treatment to remove unwanted metals.
Uses of Sodium Bicarbonate
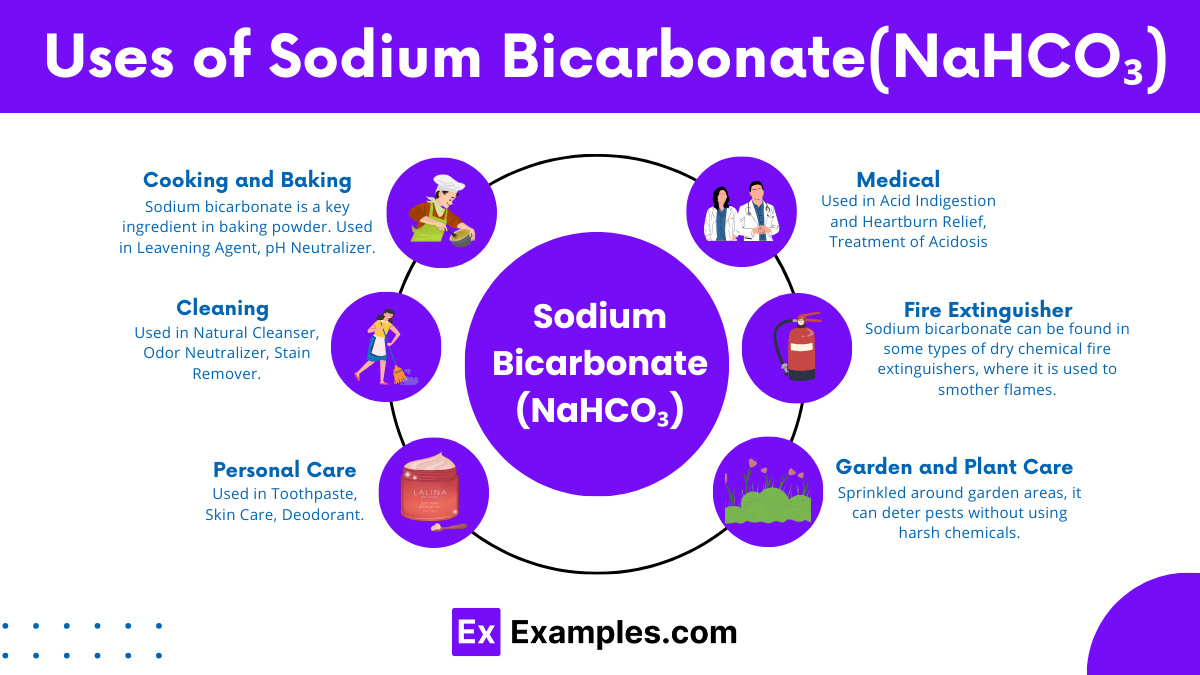
Cooking and Baking
- Leavening Agent: Sodium bicarbonate is a key ingredient in baking powder. When combined with an acid, it produces carbon dioxide gas, causing dough and batters to rise and become light and fluffy.
- pH Neutralizer: It can neutralize excess acidity in tomatoes or in the cooking water of beans, improving flavor and reducing cooking time.
Cleaning
- Natural Cleanser: Its mild abrasive properties make it an effective cleaner for pots, pans, sinks, and other surfaces without scratching them.
- Odor Neutralizer: It can absorb and neutralize odors in refrigerators, carpets, and trash cans.
- Stain Remover: Mixed into a paste with water, it can lift stains from fabric, plastic, and enamel surfaces.
Personal Care
- Toothpaste: Sodium bicarbonate can be used as a toothpaste or added to toothpaste to gently remove stains, whiten teeth, and neutralize bad breath.
- Skin Care: A baking soda bath can soothe itchy skin, and a paste can help relieve insect bites and minor rashes.
- Deodorant: Its odor-absorbing qualities make it a simple and effective underarm deodorant.
Medical Uses
- Acid Indigestion and Heartburn Relief: Sodium bicarbonate can quickly neutralize stomach acid, providing relief from acid indigestion and heartburn.
- Treatment of Acidosis: In medical settings, it is used to treat acidosis, helping to balance the pH of the blood in critical situations.
Fire Extinguisher
- Fire Suppression: Sodium bicarbonate can be found in some types of dry chemical fire extinguishers, where it is used to smother flames.
Garden and Plant Care
- Fungicide: A solution of sodium bicarbonate can act as a fungicide to protect plants from fungal infections.
- Pest Control: Sprinkled around garden areas, it can deter pests without using harsh chemicals.
Miscellaneous
- Pool Maintenance: It is used to maintain the pH level and alkalinity of pool water, ensuring a safe and comfortable swimming environment.
- Leather Care: Mixed with water, it can clean leather furniture and accessories, removing dirt and oil buildup.
Benefits of Sodium Bicarbonate
Health and Medical
Relieves heartburn and acid indigestion, improves athletic performance by buffering lactic acid, and promotes oral health by whitening teeth and neutralizing bad breath.
Household Cleaning
Acts as an eco-friendly cleaning agent for various surfaces, neutralizes odors, and gently removes stains without scratching.
Personal Care
Soothes itchy skin and bug bites, and serves as a natural deodorant.
Environmental
Safe for the environment, breaking down into harmless components.
Cooking and Food Preparation
Works as a leavening agent in baking, improves the texture and flavor of foods.
Cost-Effectiveness
Affordable and reduces the need for multiple specialized products.
Safe Use
Gentle on most surfaces and fabrics, offering a safe cleaning option.
Side Effects Of Sodium Bicarbonate
Electrolyte Imbalance
Can disrupt sodium and potassium levels, leading to cramping and irregular heartbeats.
Metabolic Alkalosis
Raises blood pH, causing confusion, twitching, and nausea.
Gastrointestinal Issues
May result in stomach cramps, gas, and diarrhea.
High Blood Pressure
Its sodium content can elevate blood pressure, a concern for those with hypertension.
Kidney Stress
Long-term or excessive use can strain or damage the kidneys.
Respiratory Effects
Inhalation can cause coughing and shortness of breath due to irritation of mucous membranes.


