What is the chemical symbol for molybdenum?
Mo
Mn
Mg
Mb
Discover the multifaceted world of Molybdenum, a remarkable element crucial for industrial applications and scientific advancements. This guide delves into the definition, meaning, and extensive uses of Molybdenum, alongside a comprehensive look at its compounds. Uncover how Molybdenum enhances material strength, contributes to high-performance alloys, and plays a pivotal role in chemical reactions. Whether in technology, medicine, or environmental solutions, Molybdenum’s versatility and importance cannot be overstated. Join us to explore its unique characteristics and pivotal contributions across various sectors.
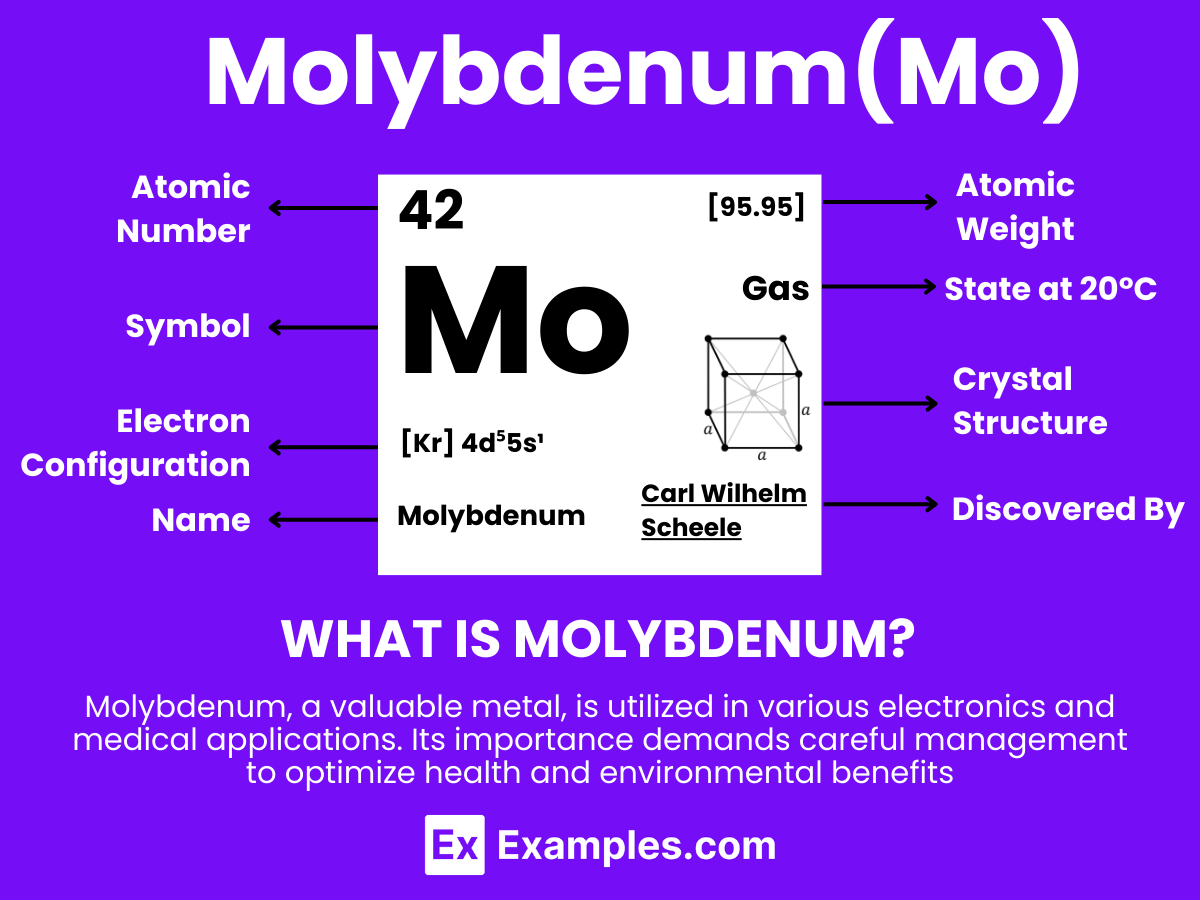
Molybdenum is a chemical element with the symbol Mo and atomic number 42. It is a transition metal known for its shiny, silver-gray appearance and high melting point. Molybdenum exhibits excellent strength at high temperatures, resistance to corrosion, and does not easily react with air or water, making it highly stable under various conditions. Historically, Molybdenum has been used to improve steel alloys, and today, it is crucial in the manufacturing of high-strength steels, chemical applications, and electronic devices.
Molybdenum is not commonly found or used in its gaseous state due to its high melting and boiling points. Molybdenum is a solid metal under standard conditions. However, I can explain the atomic structure of Molybdenum as an element, which applies to all its physical states (solid, liquid, gas) when referring to its electrons, protons, and neutrons.
Molybdenum (Mo) has an atomic number of 42, meaning it possesses 42 protons in its nucleus. The number of neutrons in the most abundant isotope of Molybdenum, Molybdenum-98, is 56, giving it a mass number of 98 (42 protons + 56 neutrons). The electrons are arranged in orbitals around the nucleus. The electron configuration of Molybdenum is [Kr] 4d⁵ 5s¹, indicating it has one electron in the 5s orbital and five electrons in the 4d orbital beyond the filled orbitals of Krypton (Kr), a noble gas.
| Property | Value |
|---|---|
| Atomic Number | 42 |
| Atomic Mass | 95.95 amu |
| Density | 10.28 g/cm³ at 20°C |
| Melting Point | 2,623°C (4,753°F) |
| Boiling Point | 5,560°C (10,040°F) |
| Atomic Volume | 9.4 cm³/mol |
| State at 20°C | Solid |
| Color | Silvery-gray |
| Crystal Structure | Body-centered cubic (BCC) |
| Electrical Resistivity | 5.34 μΩ·cm at 20°C |
| Thermal Conductivity | 138 W/(m·K) at 20°C |
| Thermal Expansion | 4.8 × 10⁻⁶ K⁻¹ at 25°C |
| Young’s Modulus | 329 GPa |
| Shear Modulus | 126 GPa |
| Bulk Modulus | 230 GPa |
| Mohs Hardness | 5.5 |
| Magnetic Ordering | Paramagnetic |
| Property | Value |
|---|---|
| Melting Point | 1855°C (3371°F) |
| Boiling Point | 4409°C (7968°F) |
| Heat of Fusion | 14 kJ/mol |
| Heat of Vaporization | 580 kJ/mol |
| Specific Heat Capacity | 0.278 J/(g·K) |
| Property | Value |
|---|---|
| Density | 10.28 g/cm³ |
| Mohs Hardness | 5.5 |
| Tensile Strength | Varies with alloy and heat treatment |
| Ductility | Moderate, improves with purity |
| Malleability | High at high temperatures |
| Property | Value |
|---|---|
| Electrical Conductivity | Good, approximately 30% IACS |
| Magnetic Susceptibility | Paramagnetic at room temperature |
| Superconductivity | Becomes superconducting below 0.915 K |
| Property | Value |
|---|---|
| Natural Isotopes | Mo-92, Mo-94, Mo-95, Mo-96, Mo-97, Mo-98, Mo-100 |
| Neutron Cross Section | High for Mo-95 |
| Common Use in Nuclear Industry | Mo-98 is used for producing medical isotope Tc-99m |
The preparation of Molybdenum typically involves the extraction and purification of molybdenum from its primary ore, molybdenite (MoS₂). The process encompasses several crucial steps:
| Isotope | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|
| Mo-92 | 14.84 | Stable | — |
| Mo-94 | 9.25 | Stable | — |
| Mo-95 | 15.92 | Stable | — |
| Mo-96 | 16.68 | Stable | — |
| Mo-97 | 9.55 | Stable | — |
| Mo-98 | 24.13 | Stable | Common target for Tc-99m production |
| Mo-100 | 9.63 | 7.8×10^18 years (theoretical) | Used in research for double beta decay |
Molybdenum plays a crucial role in various industrial, chemical, and medical applications due to its unique properties. Some of the primary uses include:
The production of molybdenum involves several steps, from mining the primary ore, molybdenite (MoS₂), to refining the metal into its pure form or specific compounds. Here’s an overview of the key processes involved in the production of molybdenum:
Molybdenum’s unique properties, including its high melting point, strength, corrosion resistance, and ability to form stable compounds, make it useful in a wide range of applications:
Molybdenum, with its unique chemical properties, is vital in various industries, enhancing material strength, corrosion resistance, and high-temperature performance. Its role in steel production, chemical catalysis, electronics, and lubrication underscores its significance. As a crucial element in both technology and biology, molybdenum continues to be indispensable in advancing modern applications and innovations.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for molybdenum?
Mo
Mn
Mg
Mb
In which group of the periodic table is molybdenum found?
Group 4
Group 5
Group 6
Group 7
Molybdenum is primarily used in which industry?
Textile
Electronics
Steel and alloys
Food processing
What is the melting point of molybdenum?
1280°C
1750°C
2623°C
3410°C
Which enzyme contains molybdenum as a cofactor?
Catalase
Nitrogenase
Amylase
Lipase
What is the common oxidation state of molybdenum in its compounds?
+2
+6
+7
+8
Molybdenum is most commonly found in which type of geological deposits?
Sedimentary
Igneous
Metamorphic
Hydrothermal
Which mineral is the primary source of molybdenum?
Bauxite
Scheelite
Molybdenite
Hematite
Molybdenum is an essential trace element for which type of organism?
Plants
Animals
Bacteria
All of the above
Which alloy is commonly formed using molybdenum?
Brass
Stainless steel
Bronze
Pewter
Before you leave, take our quick quiz to enhance your learning!

