What is the atomic number of Calcium?
18
19
20
21
Calcium, an essential element in education, plays a pivotal role in both natural processes and classroom discussions. This comprehensive guide aims to enlighten teachers about calcium’s diverse applications, ensuring they can effectively impart knowledge to their students. From understanding its fundamental properties to exploring its real-world significance, this resource provides educators with practical examples and teaching strategies, enriching the learning experience for their students. Delve into the world of calcium, and discover how to make your lessons more engaging and informative.
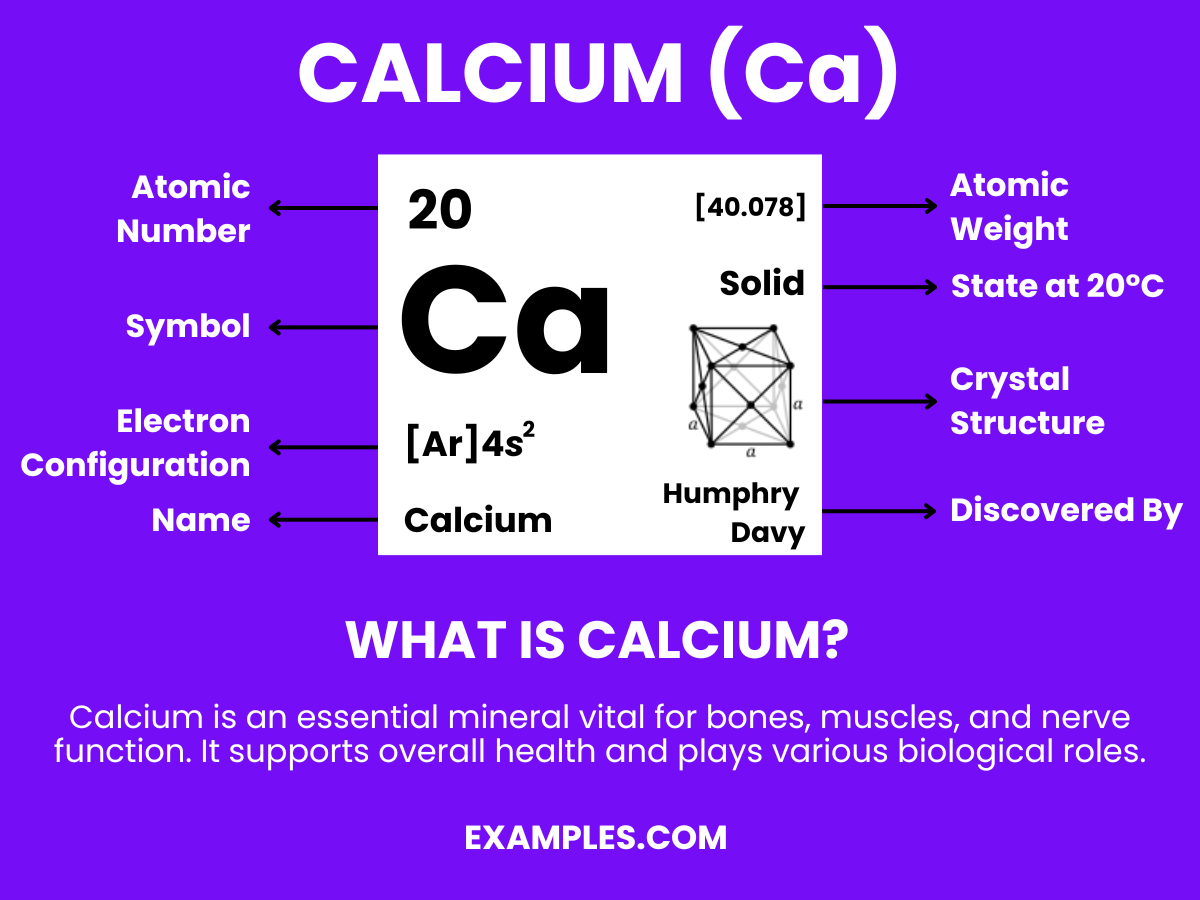
Calcium, a chemical element represented by the symbol Ca and atomic number 20, is a vital mineral for life. It’s predominantly known for its crucial role in building and maintaining strong bones and teeth. However, its importance extends beyond this, as calcium is also essential in blood clotting, muscle contraction, and nerve function. In a classroom setting, understanding calcium’s multifaceted role in both the human body and the environment provides a rich context for engaging scientific discussions. This simple yet comprehensive definition allows teachers to effectively introduce calcium in various educational scenarios, fostering a deeper understanding among students.
| Beryllium(Be) |
| Magnesium(Mg) |
| Strontium(Sr) |
| Barium(Ba) |
| Radium(Ra) |
| Property | Description |
|---|---|
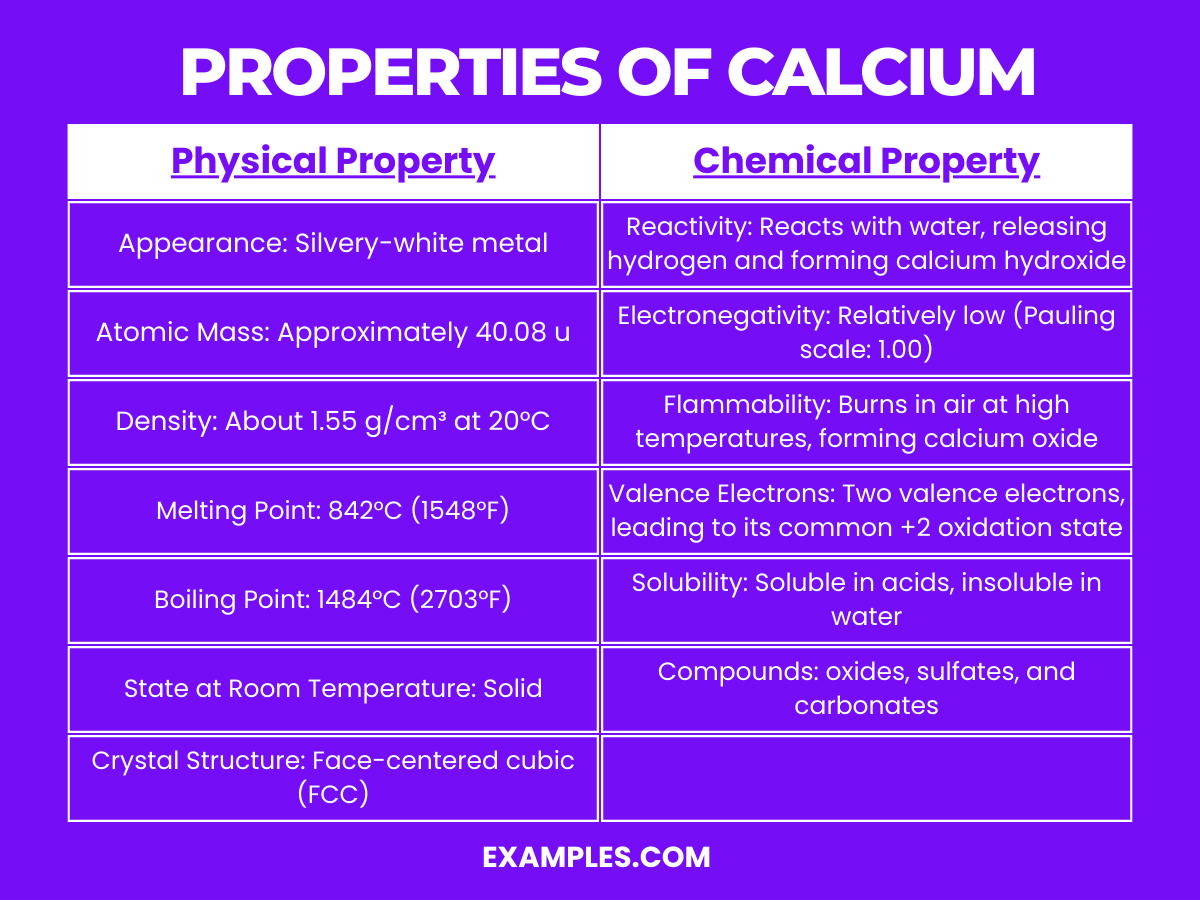
| Appearance | Silvery-white, somewhat soft metal |
| Melting Point | Approximately 842°C (1548°F) |
| Boiling Point | About 1484°C (2703°F) |
| Density | 1.55 g/cm³ at 20°C |
| State at Room Temperature | Solid |
| Malleability | Fairly malleable |
| Electrical Conductivity | Good conductor of electricity |
| Solubility | Reacts with water, insoluble in most organic solvents |
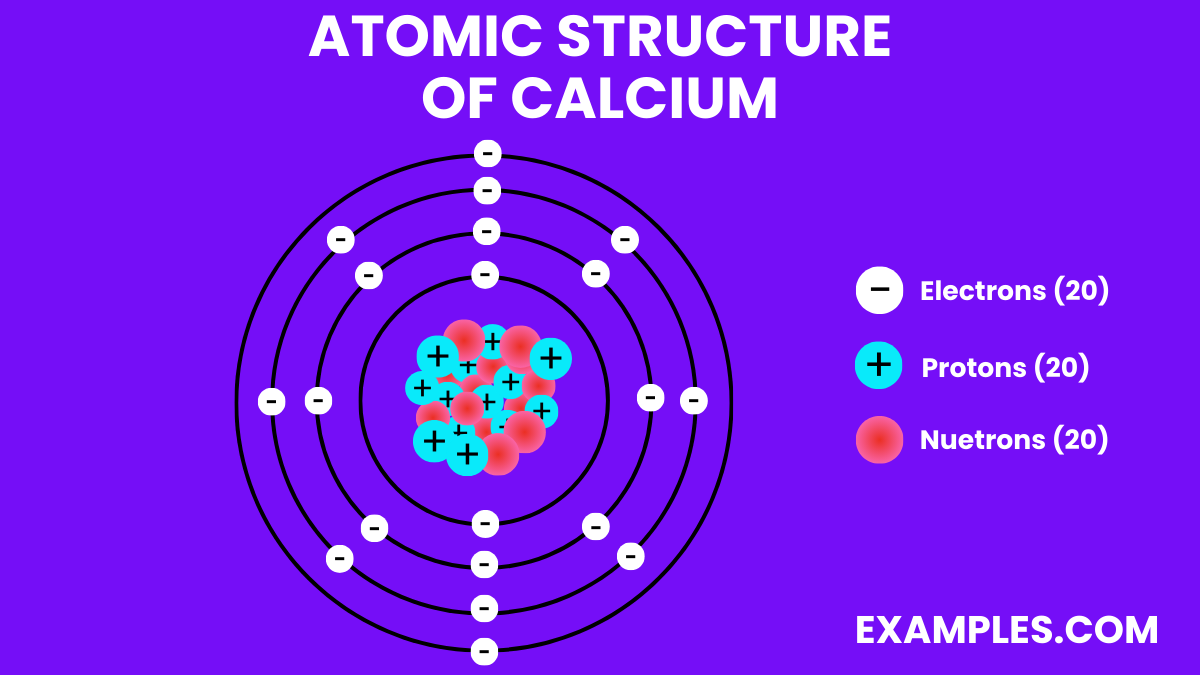
Calcium, with the atomic number 20 and symbol ‘Ca’, is a highly reactive, alkaline earth metal. It’s the fifth most abundant element in the Earth’s crust and is essential for living organisms, particularly in cell physiology.
| Property | Value with Unit |
|---|---|
| Boiling Point | 1484 °C |
| Melting Point | 842 °C |
| Heat of Vaporization | 154.7 kJ/mol |
| Heat of Fusion | 8.54 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.647 J/g·K |
| Thermal Conductivity | 201 W/m·K |
| Critical Temperature | Not Available |
| Property | Value with Unit |
|---|---|
| Density | 1.55 g/cm³ (at 20°C) |
| Viscosity | Not Applicable (Solid) |
| Solubility in Water | Reacts (Forms Ca(OH)2) |
| Color | Silvery-white |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 3.36 µΩ·m |
| Thermal Conductivity | 201 W/m·K |
| Electronegativity (Pauling scale) | 1.00 |
| First Ionization Energy | 6.1132 eV |
| Property | Value with Unit |
|---|---|
| Atomic Number | 20 |
| Atomic Mass | 40.078 amu |
| Isotopes | ^40Ca (96.941%), ^42Ca, ^43Ca, ^44Ca, ^46Ca, ^48Ca |
| Natural Abundance (for ^40Ca) | 96.941% |
| Nuclear Spin (for ^43Ca) | 7/2ℏ |
| Neutron Cross Section (for ^40Ca) | 0.41 barns |
| Nuclear Magnetic Moment (for ^43Ca) | -1.3173 µN |
| Isotope | Description |
|---|---|
| Calcium-40 | The most abundant isotope, stable, making up about 97% of natural calcium. |
| Calcium-42 | Stable isotope, comprising about 0.6% of natural calcium. |
| Calcium-43 | Stable isotope, used in medical and scientific research. |
| Calcium-44 | Another stable isotope, comprises about 2% of natural calcium. |
| Calcium-48 | The heaviest stable isotope, rare but important in scientific research for its unusual nuclear properties. |
Calcium is primarily produced through the electrolysis of fused calcium chloride (CaCl₂). The process involves several key steps:
This method is widely used because it produces high-purity calcium. However, it requires high energy inputs, making it an energy-intensive process. The demand for calcium and its compounds in various industries justifies the energy and resource investment in its production.
Calcium is crucial for maintaining overall health, but it must be consumed in balanced amounts. Here’s an overview of its health effects:
Overall, a balanced intake of calcium is vital for health, and its consumption should be carefully managed.
Calcium in the environment is generally considered non-toxic and is a naturally abundant element. However, its compounds and usage can have various environmental impacts:
Calcium is a chemical element, symbol Ca, atomic number 20, essential for living organisms, particularly in cell physiology and bone formation.
Calcium is not made from anything; it’s a naturally occurring element, extracted from minerals like limestone, gypsum, and fluorite.
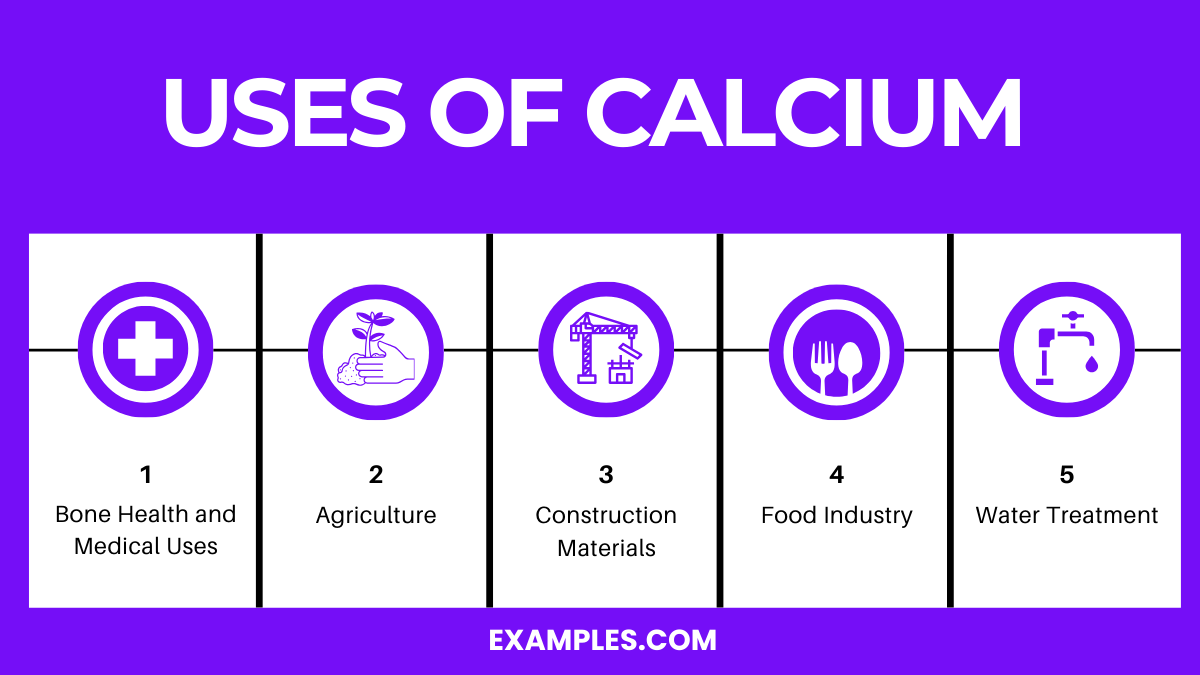
Elemental calcium is used in the production of cement, steel, and alloys, and is crucial in medical supplements and agricultural lime.
Calcium is beneficial due to its essential role in biological processes, including bone health, muscle function, and cellular signaling, and its industrial applications.
Calcium is vital for health, particularly in bone and dental strength. Its presence in various foods and supplements makes it easily accessible. Understanding calcium’s role and ensuring adequate intake are crucial for maintaining overall health and preventing bone-related disorders.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Calcium?
18
19
20
21
What is the chemical symbol for Calcium?
Ca
Cl
C
Cs
In which group of the periodic table is Calcium found?
Group 1
Group 2
Group 3
Group 4
Which of the following is a common use for Calcium?
Food preservation
Paint manufacturing
Textile industry
Fertilizer production
What is the primary mineral source of Calcium?
Bauxite
Gypsum
Calcite
Galena
What is the common oxidation state of Calcium in its compounds?
+1
+2
+3
+4
Calcium plays an important role in the human body. Which function is it primarily associated with?
Oxygen transport
Muscle contraction
DNA synthesis
Enzyme activity
What is the most abundant Calcium compound found in bones and teeth?
Calcium carbonate
Calcium sulfate
Calcium phosphate
Calcium chloride
What is the melting point of Calcium?
842°C
950°C
1115°C
1550°C
Which of the following is a property of Calcium metal?
Brittle
Non-reactive
High density
Highly malleable
Before you leave, take our quick quiz to enhance your learning!

