What is the atomic number of Barium?
56
57
55
54
Barium plays a vital role in various scientific applications, intriguing both teachers and students alike. This guide aims to demystify Barium, highlighting its unique properties and practical uses in a classroom setting. By understanding Barium’s characteristics and applications, educators can enrich their teaching methodologies and spark students’ interest in chemistry. This comprehensive overview will provide teachers with the necessary tools to effectively incorporate Barium into their curriculum, ensuring a dynamic and engaging learning experience.
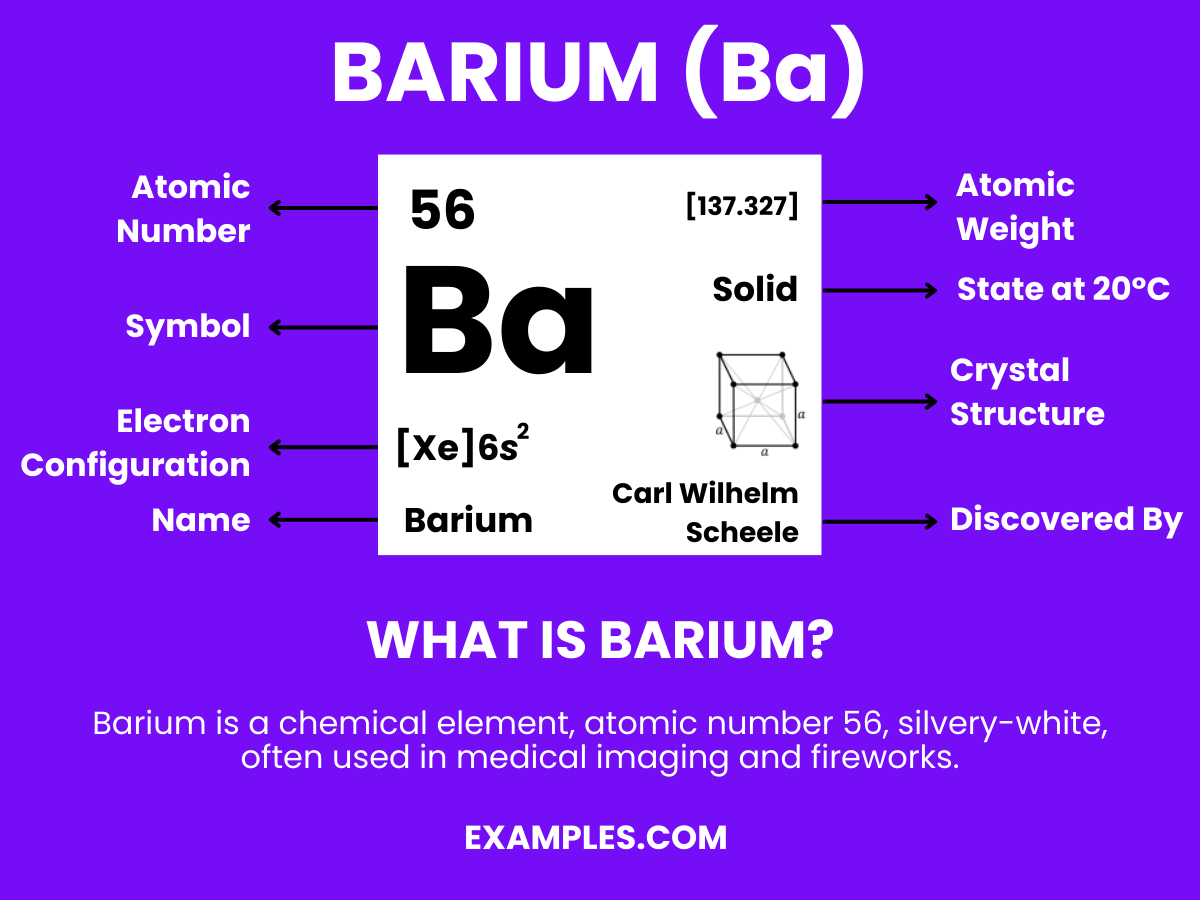
Barium is a chemical element with the symbol Ba and atomic number 56. It is a soft, silvery alkaline earth metal that, because of its high chemical reactivity, is never found in nature as a free element. Barium has several important applications, including in fireworks to create green colors, in oil and gas drilling fluids, and as a contrast agent in medical radiology. Barium compounds, such as barium sulfate and barium carbonate, are also used in the manufacturing of ceramics and glasses. Understanding Barium’s properties and uses can be a valuable addition to science education, particularly in demonstrating real-world chemical applications to students.
| Beryllium(Be) |
| Magnesium(Mg) |
| Calcium(Ca) |
| Strontium(Sr) |
| Radium(Ra) |
| Property | Description |
|---|---|
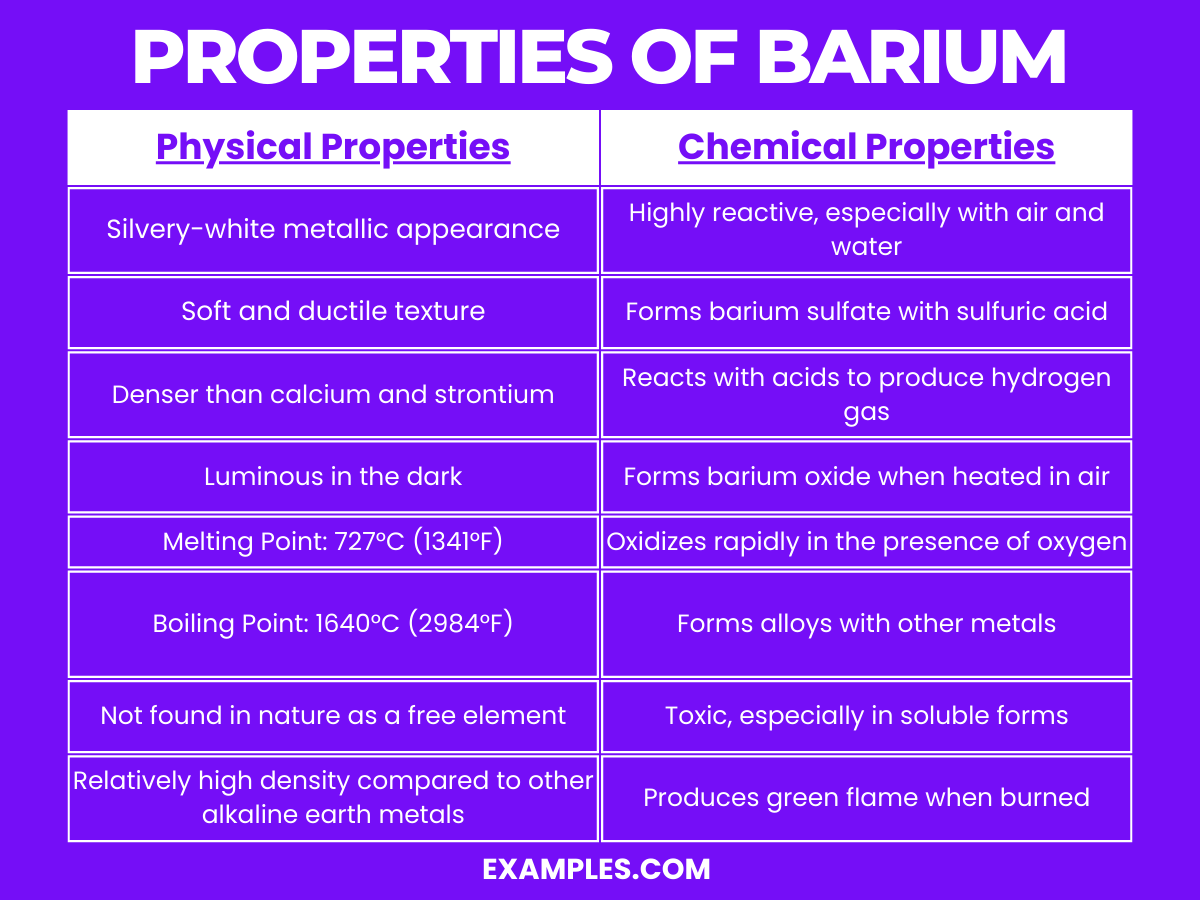
| Appearance | Silvery-white, lustrous metal |
| State at Room Temperature | Solid |
| Melting Point | 727°C |
| Boiling Point | 1897°C |
| Density | 3.51 g/cm³ |
| Malleability | Relatively soft, can be cut with a knife |
| Conductivity | Good electrical and thermal conductor |
| Solubility in Water | Insoluble, reacts with water to form barium hydroxide |
Barium is a chemical element with the symbol Ba and atomic number 56. It is part of the alkaline earth metal group, similar to calcium and magnesium, and exhibits several notable chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | 727°C (1341°F) |
| Boiling Point | 1845°C (3353°F) |
| Thermal Conductivity | 18.4 W/(m·K) |
| Specific Heat | 0.204 J/(g·K) at 25°C |
| Heat of Vaporization | 140.3 kJ/mol at boiling point |
| Heat of Fusion | 7.12 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 3.62 g/cm³ at 20°C |
| Young’s Modulus | 13 GPa |
| Tensile Strength | 13 MPa |
| Mohs Hardness | 1.25 |
| Elastic Modulus | 9.6 GPa |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 3.4 MS/m |
| Property | Description / Value |
|---|---|
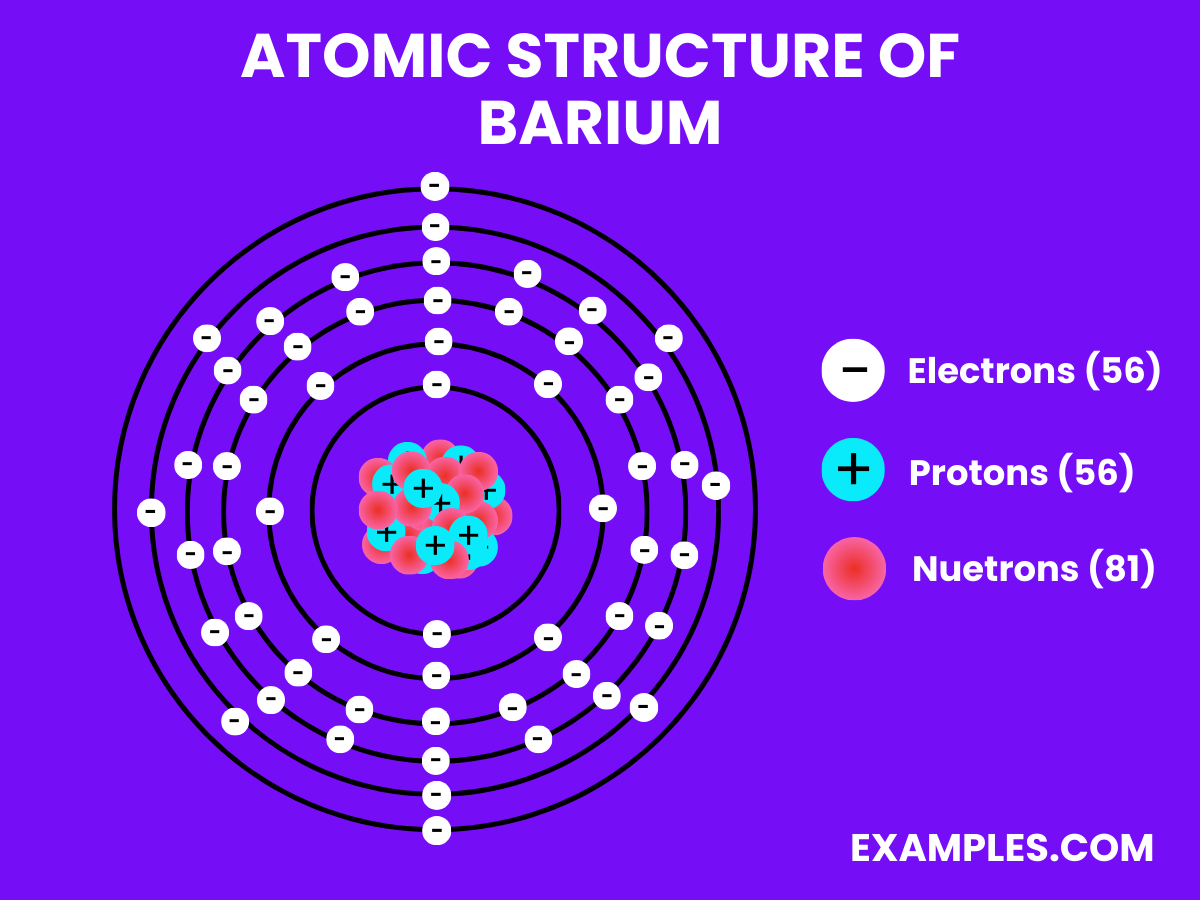
| Atomic Number | 56 |
| Atomic Mass | 137.327 u |
| Neutron Cross Section | 1.1 barns (for ^138Ba) |
| Isotopes | Natural barium is a mix of seven isotopes: ^130Ba, ^132Ba, ^134Ba, ^135Ba, ^136Ba, ^137Ba, and ^138Ba |
| Radioactivity | ^131Ba, ^133Ba, ^137mBa are among the notable radioactive isotopes, with ^137mBa being commonly used in medical and industrial applications |
Barium, a chemical element with diverse applications, forms several important compounds. Here are the top six barium compounds:
| Isotope | Symbol | Atomic Mass (u) | Half-Life |
|---|---|---|---|
| Barium-130 | ¹³⁰Ba | 129.9063 | Stable |
| Barium-132 | ¹³²Ba | 131.9051 | Stable |
| Barium-134 | ¹³⁴Ba | 133.9045 | Stable |
| Barium-135 | ¹³⁵Ba | 134.9057 | Stable |
| Barium-136 | ¹³⁶Ba | 135.9046 | Stable |
| Barium-137 | ¹³⁷Ba | 136.9058 | Stable |
| Barium-138 | ¹³⁸Ba | 137.9052 | Stable |
The most common isotope, Barium-138, accounts for approximately 71.7% of natural barium.
These isotopes are primarily used in scientific research and have various industrial applications.
The commercial production of barium involves several stages, primarily focusing on extracting barium from barite, a naturally occurring barium sulfate mineral.
The commercial production of barium must adhere to environmental and safety regulations due to the toxicity of some barium compounds. Handling and disposal of barium waste require careful management to prevent environmental contamination.
The health effects of barium depend on its forms and exposure levels. Barium compounds, when not handled properly, can pose health risks.
Barium exposure is usually occupational and related to the mining, processing, or handling of barium compounds. Safety measures and regulatory guidelines are essential to minimize health risks.
Barium compounds can impact the environment, especially when released from industrial processes or improper waste disposal.
Barium can cause gastrointestinal issues, respiratory problems, and affect heart rhythm when ingested in soluble forms. Barium sulfate used in medical imaging is generally safe.
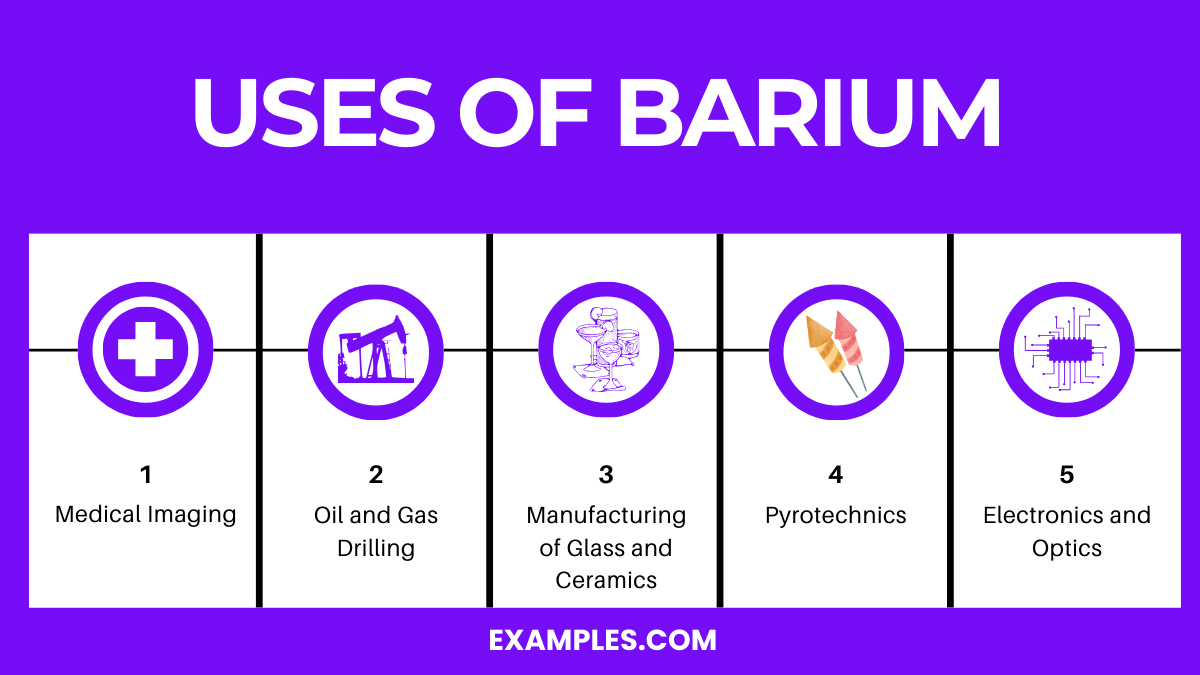
Barium is used in medical imaging, manufacturing glass and ceramics, oil and gas drilling, pyrotechnics for fireworks, and in electronics and optics.
Soluble barium compounds can be toxic, causing cardiovascular, respiratory, and neurological issues. However, barium sulfate used in medical procedures is not absorbed and is considered safe.
Foods generally have low barium levels. However, Brazil nuts, seaweed, fish, and certain leafy vegetables can contain higher amounts of naturally occurring barium.
Barium’s wide-ranging applications, from medical imaging to manufacturing, demonstrate its utility. While certain forms can be toxic, its regulated use in industries and healthcare is beneficial. Acknowledging both the uses and risks of barium is crucial for its effective and safe application.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Barium?
56
57
55
54
What is the main hazard associated with barium if ingested in soluble forms?
It is radioactive.
It is toxic.
It is an irritant.
Barium is commonly used in the production of:
Plastics
Ceramics
Fireworks
Pharmaceuticals
Before you leave, take our quick quiz to enhance your learning!

