What is the atomic number of Beryllium?
3
4
5
6
Beryllium, a fascinating element, is often overlooked in educational discussions. This guide aims to change that. We delve into the essence of Beryllium, exploring its unique properties and relevance in various fields. Our focus is on making this information accessible and engaging for teachers, enhancing their ability to convey complex concepts with ease. Beryllium’s multifaceted nature offers a rich context for learning, making it an invaluable addition to any curriculum.
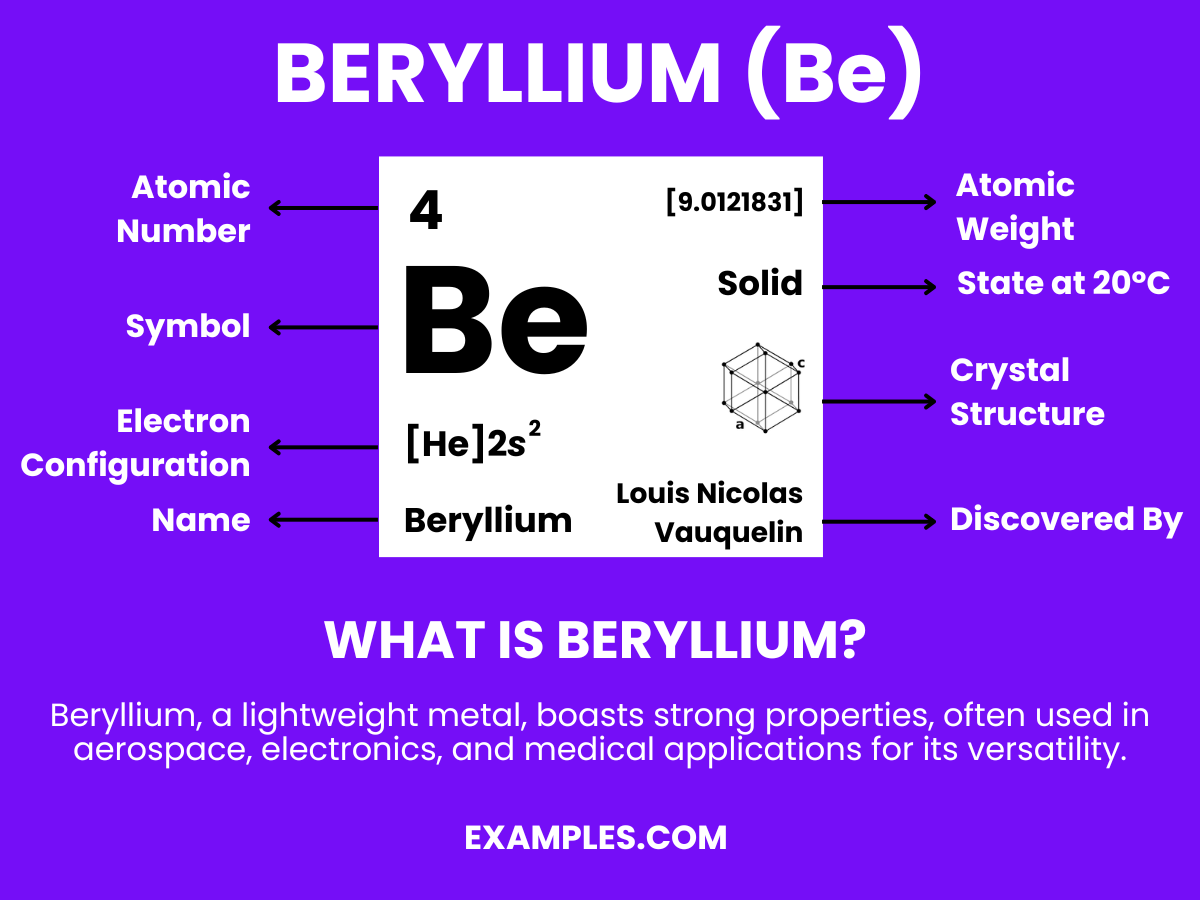
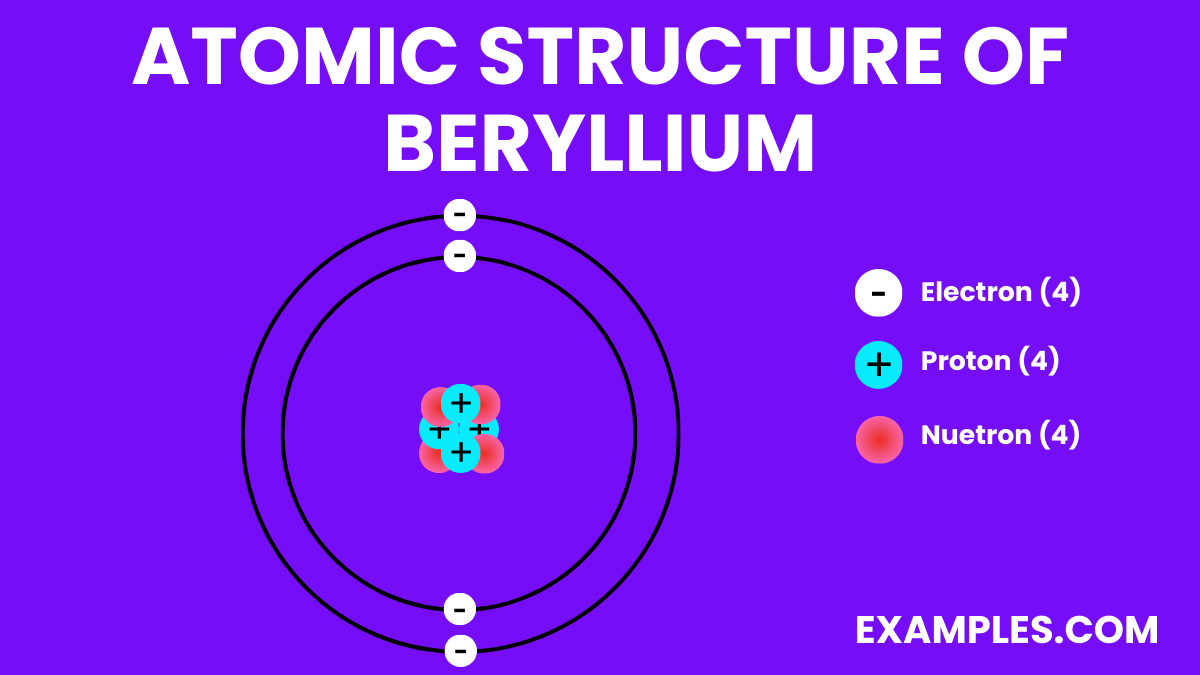
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust. This lightweight yet strong element is notable for its high melting point and excellent thermal conductivity. Beryllium is used in various applications, including aerospace, telecommunications, and nuclear reactors, due to its unique physical properties. Understanding Beryllium’s characteristics and applications can enhance teaching related to chemistry, physics, and industrial applications.
| Magnesium(Mg) |
| Calcium(Ca) |
| Strontium(Sr) |
| Barium(Ba) |
| Radium(Ra) |
| Property | Description |
|---|---|
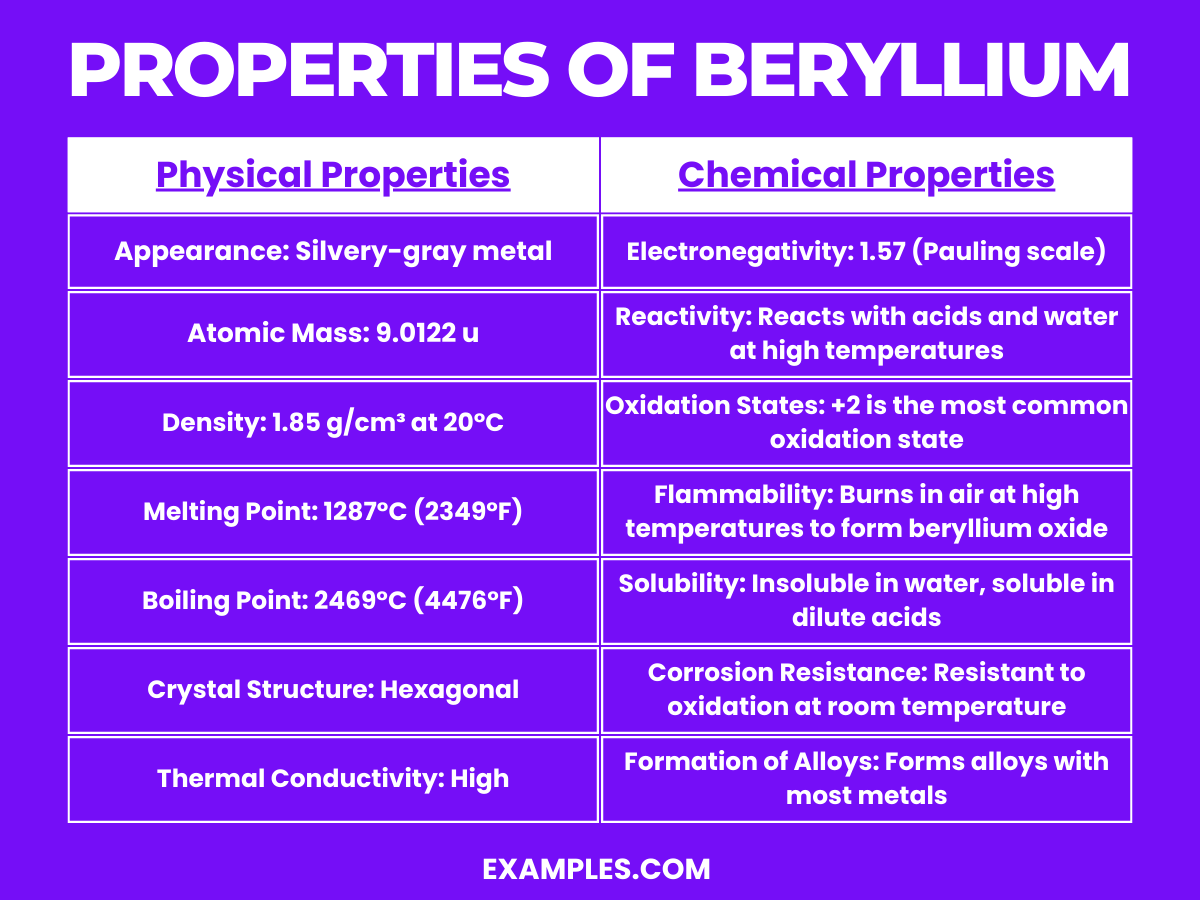
| Appearance | Silvery-gray, hard, lightweight metal. |
| Atomic Number | 4 |
| Atomic Mass | Approximately 9.0122 u |
| Density | About 1.85 g/cm³ |
| Melting Point | 1287°C (2349°F) |
| Boiling Point | 2469°C (4476°F) |
| State at Room Temperature | Solid |
| Thermal Conductivity | High thermal conductivity |
| Electrical Conductivity | Good electrical conductor, though not as much as some metals |
| Property | Description / Value |
|---|---|
| Melting Point | 1287°C (2349°F) |
| Boiling Point | 2469°C (4476°F) |
| Thermal Conductivity | 200 W/(m·K) at 25°C |
| Specific Heat | 1.82 J/(g·K) at 25°C |
| Thermal Expansion | 11.3 µm/(m·K) at 25°C |
| Property | Description / Value |
|---|---|
| Density | 1.85 g/cm³ |
| Young’s Modulus | 287 GPa |
| Tensile Strength | 370 MPa |
| Mohs Hardness | 5.5 |
| Elastic Modulus | 300 GPa |
| Property | Description / Value |
|---|---|
| Electrical Conductivity | 36% IACS (International Annealed Copper Standard) |
| Magnetic Susceptibility | -9.0·10⁻⁶ cm³/mol (diamagnetic) |
| Reflectivity | Approximately 67% at 600 nm (visible light spectrum) |
| Property | Description / Value |
|---|---|
| Atomic Number | 4 |
| Atomic Mass | 9.012182 u |
| Neutron Cross Section | 0.0092 barns (thermal neutrons) |
| Isotopes | Mainly ⁹Be; ¹⁰Be (trace, cosmogenic) |
| Radioactivity | ⁷Be (radioactive isotope, half-life: 53.22 days) |
| Isotope | Half-Life | Natural Abundance | Mode of Decay | Usage/Significance |
|---|---|---|---|---|
| Be-7 | 53.12 days | Trace | Electron capture to Li-7 | Used in radiological dating and studies. |
| Be-9 | Stable | 100% | – | The only stable isotope, used in most applications of beryllium. |
| Be-10 | 1.39 million years | Trace | Beta decay to B-10 | Used in cosmogenic radionuclide dating of soils and sediments. |
| Be-8 | 6.7×10⁻¹⁷ seconds | None (synthetic) | Alpha decay to two He-4 nuclei | Important in nuclear physics studies, particularly in demonstrating the triple-alpha process in stars. |
| Be-11 | 13.81 seconds | None (synthetic) | Beta decay to B-11 | Used in nuclear physics experiments. |
| Be-12 | 21.49 milliseconds | None (synthetic) | Beta decay to C-12 | Studied in nuclear physics for its unusual decay properties. |
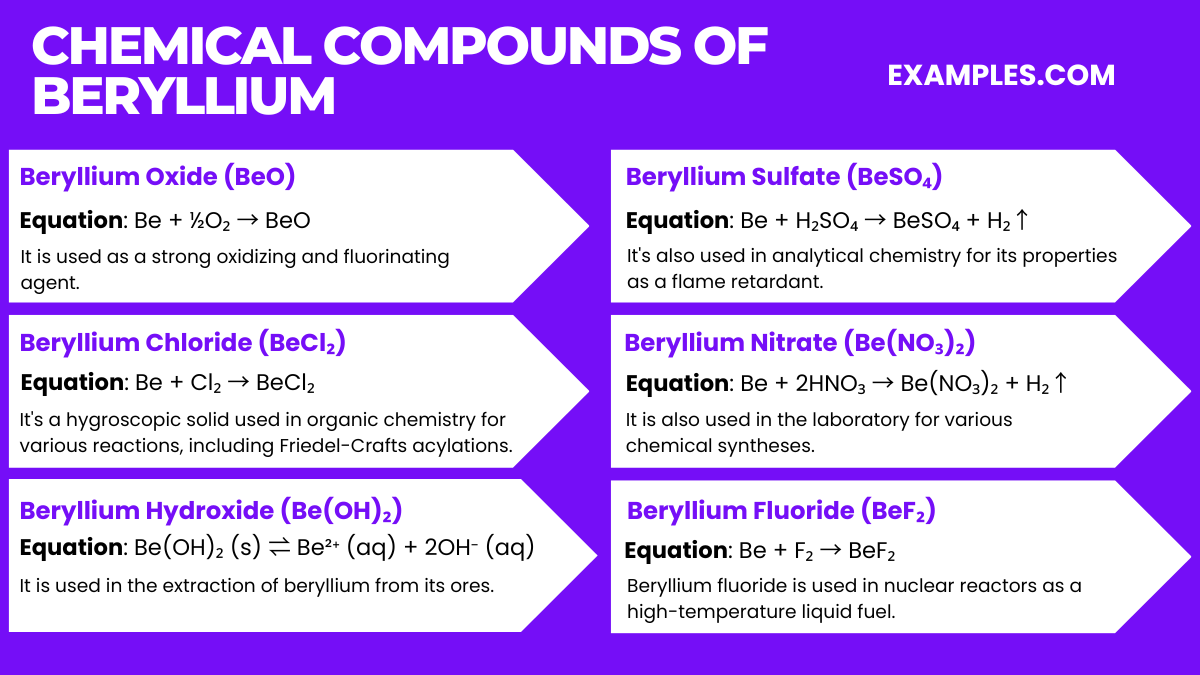
Beryllium is commercially produced primarily from the mineral beryl, which is a beryllium aluminum silicate. The process of extracting and refining beryllium from beryl involves several steps:
The final product is a lightweight metal with a high melting point, significant stiffness, and excellent thermal stability, making it valuable for various industrial applications, including aerospace, electronics, and nuclear reactors.
Beryllium has several health effects, particularly when beryllium dust or fumes are inhaled:
Proper safety measures, including protective equipment, ventilation, and exposure monitoring, are essential to minimize these health risks in workplaces where beryllium is used.
The environmental impact of beryllium is relatively limited due to its low concentration in the Earth’s crust and in water. However, there are some concerns:
Beryllium is primarily used in aerospace, telecommunications, and defense, making lightweight, strong alloys and electrical conductors.
Beryllium is toxic when inhaled as dust or fumes, causing lung diseases such as chronic beryllium disease and lung cancer.
Beryllium is found in everyday items like electronics, golf clubs, and aerospace materials due to its strength and lightweight properties.
While highly toxic, beryllium isn’t the most toxic metal; however, its inhalation is particularly dangerous due to lung disease risks.
Inhaled beryllium can damage lungs, leading to chronic beryllium disease (CBD), scarring of lung tissue, and potential lung cancer.
Beryllium is valued for improving physical properties in alloys, enhancing conductivity in electrical components, and its use in nuclear reactors.
Touching beryllium metal is not typically harmful, but inhaling its dust or fumes can cause serious lung conditions. Skin contact may cause irritation.
Understanding beryllium’s unique properties, uses, and associated health risks is crucial. When writing about beryllium, emphasize its industrial significance and toxicity precautions. Highlight its applications in various fields while underscoring the importance of safe handling. This guide offers a balanced view, blending beryllium’s benefits with its potential hazards, providing comprehensive insights for various audiences.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Beryllium?
3
4
5
6
Which property makes Beryllium useful in aerospace applications?
High reactivity
High thermal stability
High toxicity
High elasticity
Beryllium is primarily classified as a:
Metalloid
Alkali metal
Transition metal
Alkaline earth metal
Beryllium\'s ability to absorb large amounts of heat makes it suitable for use as a:
Semiconductor
Thermal conductor
Heat shield
Catalyst
Which of the following industries is NOT a typical user of beryllium?
Automotive
Jewelry making
Electronics
Nuclear
Exposure to beryllium dust can lead to a chronic disease known as:
Berylliosis
Bronchitis
Asbestosis
Silicosis
Beryllium reacts with oxygen to form:
Beryllium oxide
Beryllium chloride
Beryllium sulphate
Beryllium nitrate
Which of the following is NOT a characteristic of beryllium?
Lightweight
High melting point
Radioactive
Brittle under certain conditions
The discovery of beryllium was made by:
Antoine Lavoisier
Friedrich Wöhler
Nicolas-Louis Vauquelin
Henry Moissan
Beryllium is used in the production of:
Plastics
Ceramics
Alloys
Solvents
Before you leave, take our quick quiz to enhance your learning!

