What is the atomic number of Gallium?
30
31
32
33
Discover the fascinating world of Gallium, a versatile element with remarkable properties and wide-ranging applications. This comprehensive guide dives into Gallium’s unique characteristics, showcasing its role in modern technology, from electronics to high-temperature thermometers. Gallium’s low melting point and ability to alloy with other metals make it invaluable in various industries. Through practical examples, explore how Gallium is transforming innovations and becoming a cornerstone in fields like semiconductors and photovoltaics. Unveil the secrets of Gallium, an element that’s shaping the future of technology and beyond.
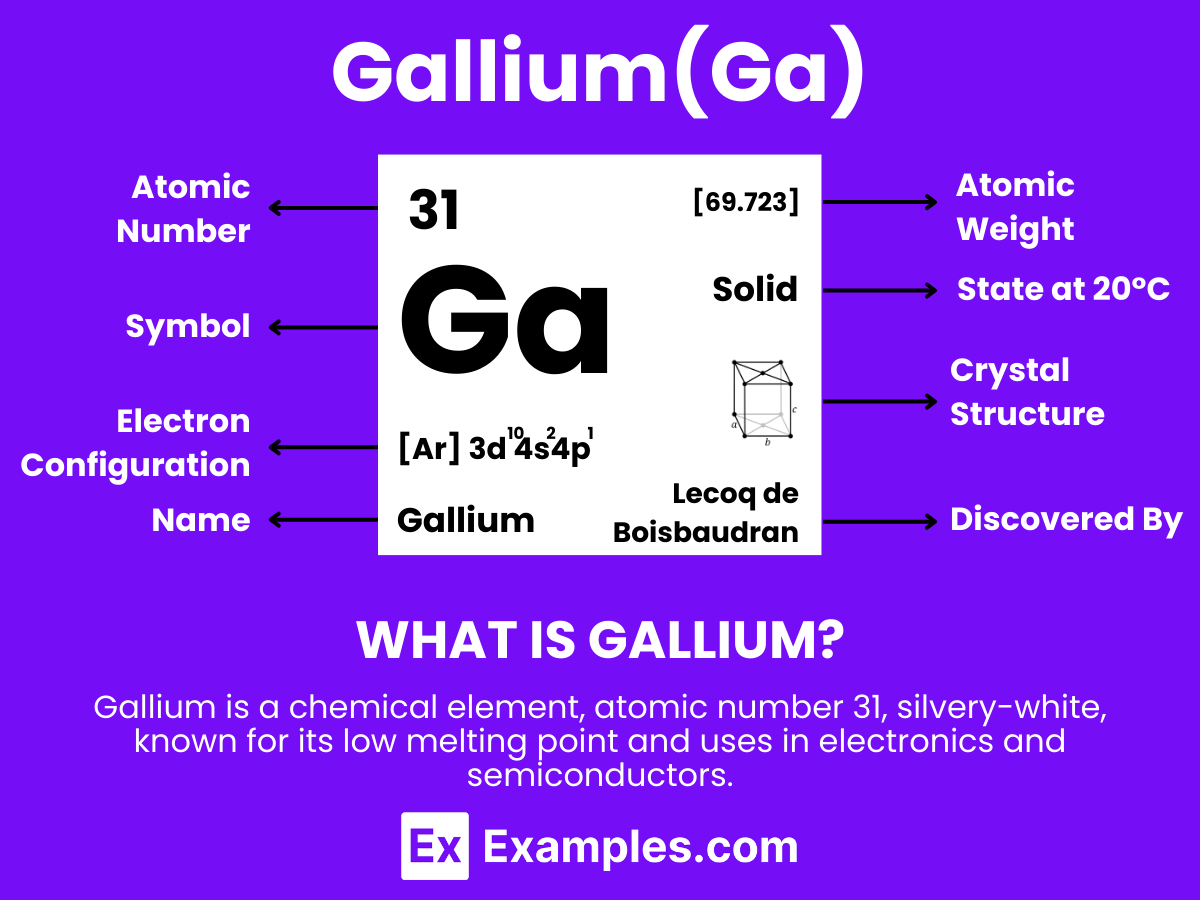
Gallium is a soft, silvery metallic element that stands out due to its unique properties and extensive range of applications. With the atomic number 31, Gallium is remarkable for its low melting point of approximately 29.76°C (85.57°F), which allows it to melt in the palm of one’s hand. This element does not occur as a free metal in nature but is extracted from minerals like bauxite and sphalerite. Gallium is utilized in various fields, notably in electronics for manufacturing semiconductors, LEDs, and solar panels. Its ability to form alloys with other metals enhances its versatility, making Gallium a critical material in scientific research, medical diagnostics, and the development of innovative technologies.
Gallium, unlike hydrogen, is a metal with distinctive characteristics, including a low melting point but a very high boiling point, indicating its preference to remain in solid or liquid form under normal conditions. Gallium’s behavior at the atomic and molecular levels is quite different from that of hydrogen due to its position in the periodic table and its metallic nature.
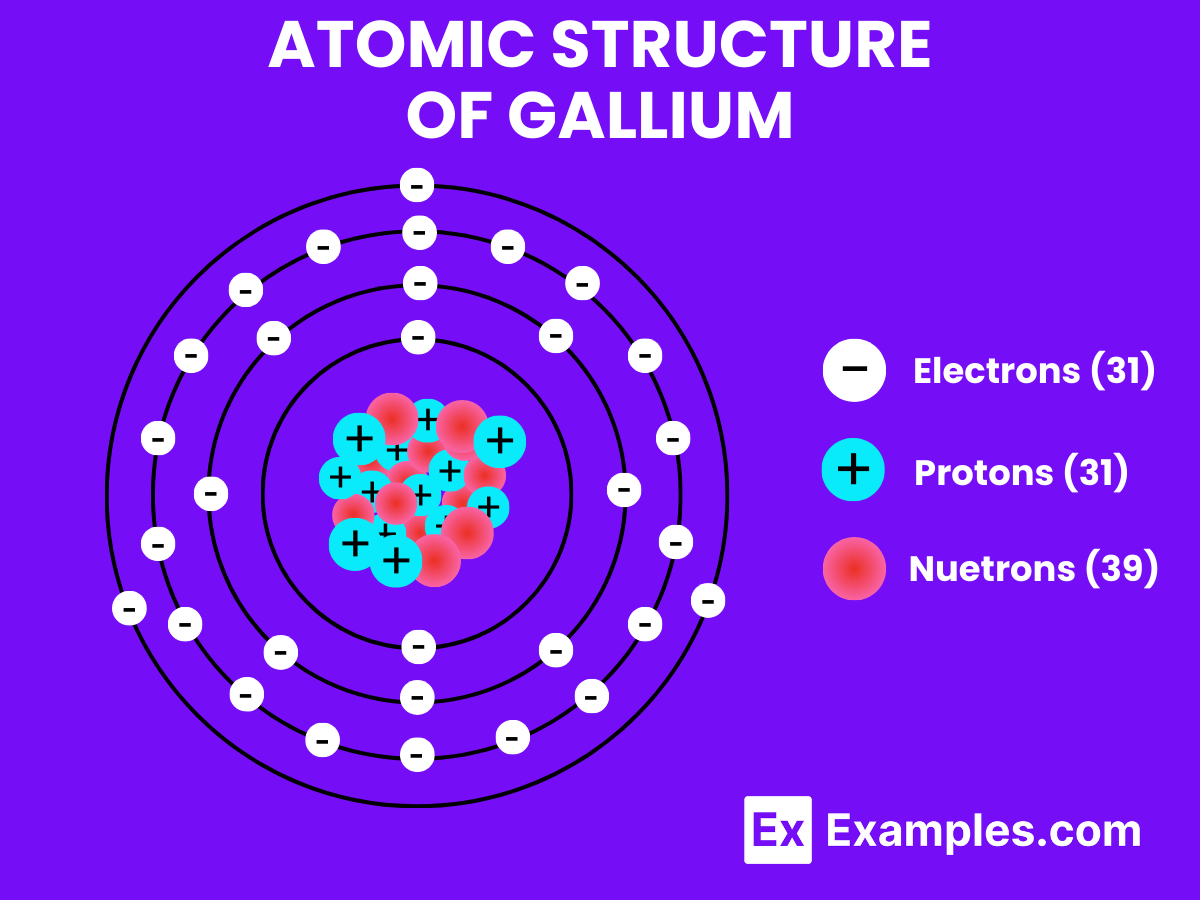
Atomic Level: Each gallium atom (Ga) contains 31 protons in its nucleus and has 31 electrons orbiting around it. The electron configuration of gallium is [Ar] 3d¹⁰ 4s² 4p¹, which means it has three electrons in its outermost shell available for bonding.
Molecular Formation: In its metallic form, gallium does not form molecules like H₂. Instead, gallium atoms are arranged in a crystalline lattice structure when solid. This structure involves the sharing of electrons between many gallium atoms in a metallic bond, which is different from the covalent bonding seen in hydrogen molecules. When melted, gallium becomes a liquid but retains its metallic bonding to some degree, leading to its high density and surface tension even in liquid form.
Gallium’s bonds within its lattice are strong, allowing it to maintain its structure until it reaches its melting point of about 29.76°C (85.57°F). Unlike hydrogen, which is a gas at room temperature, gallium is solid but easily melts into a liquid at a temperature slightly above room temperature. It does not exist naturally as a diatomic gas or in a gaseous state under normal conditions due to its high boiling point of approximately 2204°C (3999°F)
| Property | Description |
|---|---|
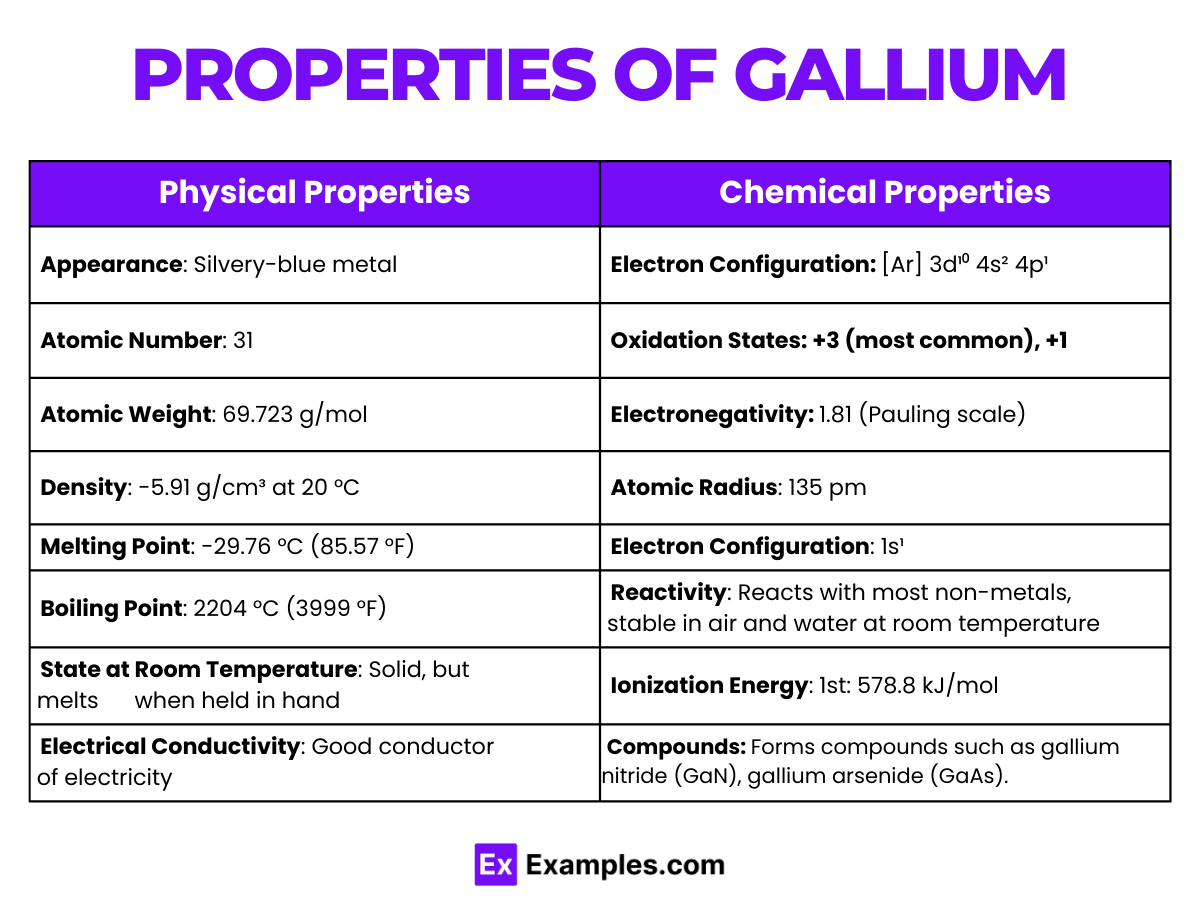
| Appearance | Silvery-white and metallic |
| Atomic Number | 31 |
| Atomic Weight | 69.723 |
| Melting Point | Approximately 29.76°C (85.57°F) |
| Boiling Point | Approximately 2204°C (3999°F) |
| Density | 5.904 grams per cubic centimeter at 29.6°C (liquid phase) |
| State at Room Temperature | Solid, but melts slightly above room temperature |
| Electron Configuration | [Ar] 3d¹⁰ 4s² 4p¹ |
| Crystal Structure | Orthorhombic at room temperature |
| Thermal Conductivity | 40.6 W/(m·K) at 300 K |
| Electrical Resistivity | 270 nΩ·m at 20°C |
| Electronegativity | 1.81 (Pauling scale) |
| Standard Atomic Weight | 69.723 |
| Phase at STP | Solid |
| Solubility | Insoluble in water, soluble in acids and alkalis |
| Common Oxidation States | +3 |
Gallium, a metal with unique characteristics, exhibits intriguing chemical behaviors. Here’s an overview of its chemical properties, supported by relevant equations to illustrate its reactivity:
| Property | Value with Unit |
|---|---|
| Boiling Point | 2204 °C |
| Melting Point | 29.76 °C |
| Critical Temperature | Not Clearly Defined |
| Critical Pressure | Not Clearly Defined |
| Heat of Vaporization | 256 kJ/mol |
| Heat of Fusion | 5.59 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.371 J/g·K |
| Thermal Conductivity | 40.6 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 29.76 °C) | 6095 kg/m³ |
| Viscosity (near m.p.) | 1.81 mPa·s |
| Solubility in Water | Insoluble |
| Color | Silvery |
| Phase at Room Temperature | Solid (melts slightly above room temp) |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 270 nΩ·m |
| Thermal Conductivity | 40.6 W/m·K |
| Magnetic Susceptibility | -0.081 × 10^-6 cm^3/mol |
| Electronegativity (Pauling scale) | 1.81 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 31 |
| Atomic Mass | 69.723 amu |
| Isotopes | ^69Ga (60.1%), ^71Ga (39.9%) |
| Nuclear Spin (for ^69Ga) | 3/2 ℏ |
| Nuclear Spin (for ^71Ga) | 3/2 ℏ |
| Neutron Cross Section (for ^69Ga) | 2.18 barns |
| Neutron Cross Section (for ^71Ga) | 3.6 barns |
| Nuclear Magnetic Moment (for ^69Ga) | 2.0165 µN |
| Nuclear Magnetic Moment (for ^71Ga) | 2.562 µN |
The preparation of gallium primarily involves extracting it from its ores since it does not occur in free form due to its reactive nature. The most common method of gallium production is through the processing of bauxite ore, which is also the principal source of aluminum. Another source is the sphalerite ore, from which zinc is produced. Here’s an overview of the steps involved in the preparation of gallium:
Regardless of the source, the gallium obtained from these processes may still contain impurities. Further purification is often required, which can be achieved through zone refining. This process involves melting a small section of a long bar of impure metal and slowly moving it along the bar. The impurities concentrate in the melted region and are moved along with the zone, leaving behind a length of purified gallium.
The purified gallium is then solidified and prepared for commercial or industrial use, depending on the required purity level. Gallium’s unique properties, particularly its low melting point and ability to form alloys, make it valuable in various applications, including electronics and high-temperature thermometers.
Gallium forms a variety of chemical compounds, many of which are significant in industrial applications, especially in electronics and optics. Below are some notable gallium compounds:
These compounds of gallium are pivotal in the development of advanced materials and technologies, underscoring the element’s versatility and importance in modern science and engineering
Gallium has two naturally occurring isotopes, gallium-69 (^(69)Ga) and gallium-71 (^(71)Ga), with distinct properties and applications in various fields. Here’s a brief overview of these isotopes:
Gallium, primarily has two stable isotopes which are significant in both natural occurrence and practical applications. Here’s an overview of these isotopes, similar to the format used for hydrogen:
| Isotope | Symbol | Protons | Neutrons | Natural Abundance | Atomic Mass | Common Uses |
|---|---|---|---|---|---|---|
| Gallium-69 | ⁶⁹Ga | 31 | 38 | ~60.1% | 68.925 | Electronics, semiconductors, NMR spectroscopy |
| Gallium-71 | ⁷¹Ga | 31 | 40 | ~39.9% | 70.924 | Electronics, semiconductors, neutrino detection |
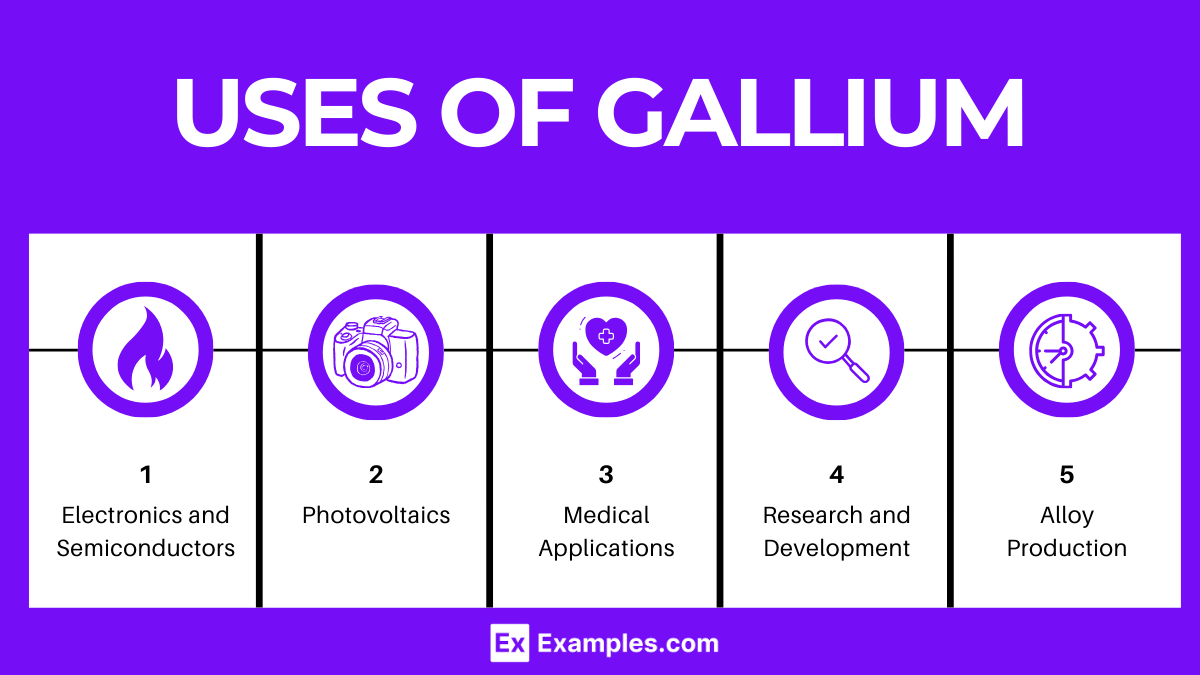
Gallium is a versatile metal with a wide range of applications, owing to its unique properties such as a low melting point, ability to alloy with other metals, and semiconductor characteristics. Here are some of the primary uses of gallium:
Gallium is crucial in the production of photovoltaic cells, particularly those made from GaAs, which are highly efficient at converting sunlight into electricity. These cells are used in space applications and in concentrated solar power installations due to their high efficiency and durability.
Gallium isotopes are used in radiopharmaceuticals for medical imaging and diagnostics. Gallium-67, for example, is used in gallium scans to detect infections and tumors.
Due to its unusual melting point (29.76°C), gallium is used in high-precision thermometers and thermal gauges, especially for temperatures close to its melting point.
Gallium can easily alloy with other metals, and these alloys have a variety of applications. For example, alloys of gallium, indium, and tin (known as Galinstan) have a low melting point and are used as mercury replacements in thermometers, barometers, and other devices.
Compounds of gallium, like gallium selenide (GaSe), are used in nonlinear optics for frequency doubling, parametric oscillation, and other applications that require the generation of coherent light at different frequencies.
Gallium’s unique properties make it a subject of ongoing research for new applications, including advanced materials and nanotechnology. Gallium liquid metal alloys are being explored for use in flexible electronics and self-healing circuits.
Gallium-71 is used in neutrino detectors for astrophysical research. It has the ability to capture neutrinos, allowing scientists to study solar and cosmic phenomena.
Gallium’s role in technology and science is expansive and continues to grow as new applications for this remarkable metal are discovered. Its contributions to electronics, renewable energy, and medical diagnostics underscore its importance in modern technology and research.
Gallium is not found in its elemental form in nature but is extracted as a byproduct of the processing of other metals, notably aluminum and zinc. The primary sources of gallium are the bauxite ore used in aluminum production and the sphalerite ore for zinc. Here’s a simplified overview of the production process:
Gallium’s unique physical and chemical properties enable its use in a variety of high-tech applications:
Gallium can weaken or damage certain metals like aluminum by infiltrating the grain boundaries, leading to structural failure without technically “destroying” the metal.
Yes, gallium is safe to touch. It is considered to have low toxicity, but prolonged skin contact should be avoided to prevent potential irritation.
Gallium, with its unique low melting point and applications in semiconductors, plays a critical role in modern technology. Safe for handling, its use in electronics, medicine, and research underscores its versatility and growing importance in advancing innovative solutions.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Gallium?
30
31
32
33
What is the chemical symbol for Gallium on the periodic table?
Ga
Gl
Gm
Gn
Gallium belongs to which group in the periodic table?
Group 11
Group 12
Group 13
Group 14
What is the melting point of Gallium?
-30°C
0°C
29.76°C
100°C
Gallium is primarily extracted from which type of ores?
Bauxite and Zinc ores
Copper and Iron ores
Lead and Silver ores
Gold and Platinum ores
Which of the following is a key application of Gallium?
Jewelry
Semiconductor industry
Paints and coatings
Agriculture
What is the electron configuration of Gallium?
[Ar] 3d10 4s2 4p1
[Ar] 3d10 4s2 4p2
[Kr] 4d10 5s2 5p1
[Ne] 3s2 3p1
Which property of Gallium allows it to be used in high-temperature thermometers?
High boiling point
Low melting point
Non-reactivity
High electrical conductivity
What color is Gallium in its pure form?
Silver
Gray
White
Blue
Gallium is known for expanding when it solidifies. What property does this attribute relate to?
Density decrease
Thermal expansion
Magnetic property
Electrical resistivity
Before you leave, take our quick quiz to enhance your learning!

