What is the primary function of hemoglobin in the blood?
Transport nutrients
Carry oxygen
Regulate blood pressure
Fight infections

Hemoglobin is a complex compound crucial in the field of chemistry and biology, primarily responsible for transporting oxygen from the lungs to various parts of the body and then carrying carbon dioxide back to the lungs to be exhaled. This protein is found in the red blood cells and gives them their characteristic red color. Understanding hemoglobin helps us grasp how oxygen reaches our vital organs and tissues, emphasizing its significant role in sustaining life. Its structure includes iron, which binds oxygen, showcasing the intricate relationship between chemical elements and biological functions.
In healthy adults, Hemoglobin A constitutes about 97% of the hemoglobin. It features two alpha and two beta chains. This structure optimally transports oxygen from the lungs to various body parts and carries carbon dioxide back to the lungs for exhalation.
Making up about 2-3% of hemoglobin in adults, Hemoglobin A2 has two alpha and two delta chains. Its presence is normal but in much smaller amounts compared to Hemoglobin A. Increased levels of HbA2 can indicate beta-thalassemia, a blood disorder.
In fetuses and newborn babies, Hemoglobin F predominates. It comprises two alpha chains and two gamma chains, enhancing oxygen capture from the mother’s bloodstream. After birth, the concentration of HbF significantly decreases and Hemoglobin A replaces it.
Hemoglobin S is a variant of hemoglobin that is associated with sickle cell disease. It arises due to a mutation in the beta chain, which causes the hemoglobin to form a sickle shape under low oxygen conditions. This abnormal shape can lead to various complications as it obstructs blood flow.
Hemoglobin C is another variant caused by a mutation in the beta chain. People with Hemoglobin C or Hemoglobin C trait usually do not have symptoms, though some might have a mild anemia. Hemoglobin C can affect the overall shape and function of red blood cells.
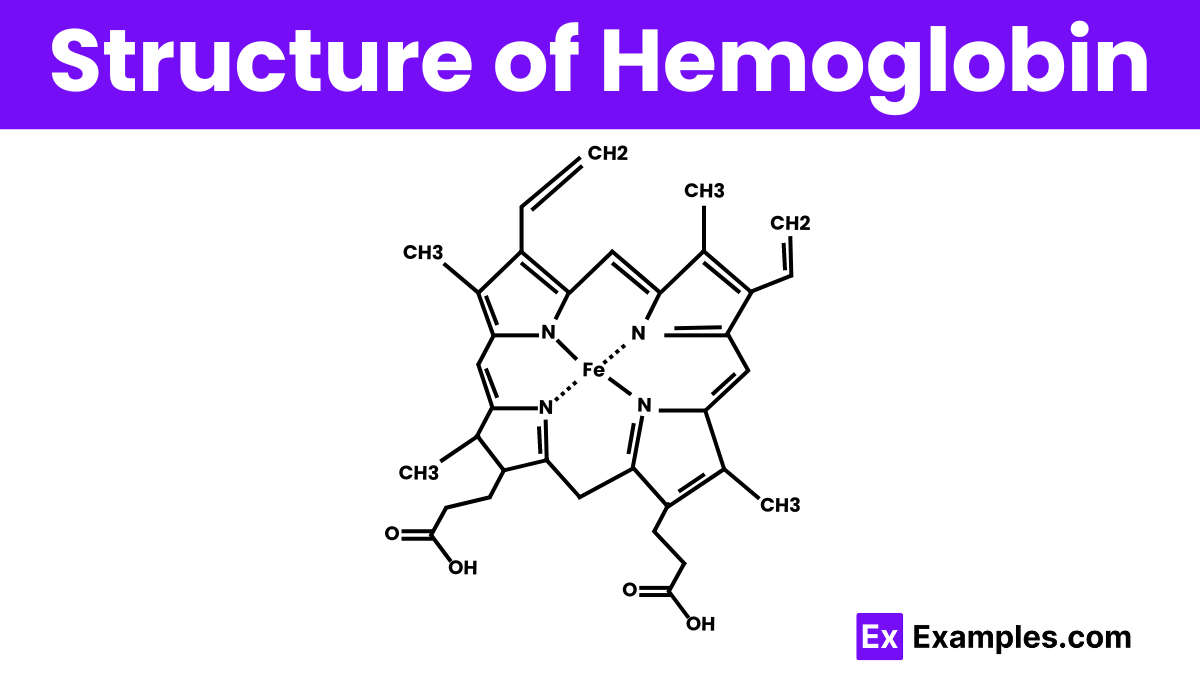
Hemoglobin is a complex protein made up of four subunits, each containing a heme group that holds an iron atom. This structure is key for its ability to bind and transport oxygen throughout the body. The four subunits are arranged in pairs: two alpha chains and two beta chains, forming a roughly spherical shape. Each iron atom in the heme groups can bind one oxygen molecule, allowing hemoglobin to efficiently pick up oxygen in the lungs and release it in tissues that need it most. This unique arrangement ensures that hemoglobin functions effectively as the body’s oxygen transporter, critical for sustaining cellular processes and overall health.
The body naturally produces hemoglobin, specifically within the bone marrow. The synthesis involves forming globin proteins and the heme group. The translation of mRNA, which encodes the alpha and beta chains of hemoglobin, produces globin. Simultaneously, the heme group synthesis occurs through a series of chemical reactions beginning with succinyl-CoA and glycine, leading to the production of a molecule called porphyrin. This porphyrin then incorporates iron to form the complete heme group. The chemical reaction is:
Once both components are available, they combine to form the complete hemoglobin molecule, ready to transport oxygen throughout the body. This synthesis is crucial for maintaining healthy oxygen levels in our tissues and organs.
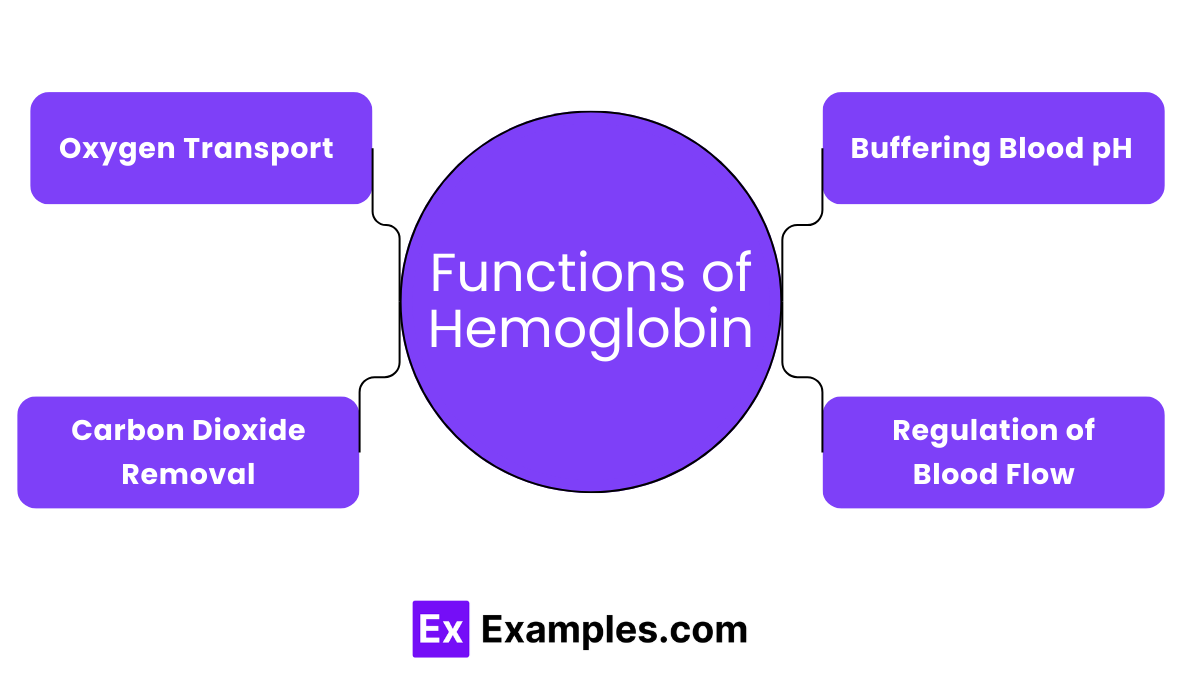
Hemoglobin primarily functions to transport oxygen from the lungs to various tissues and organs throughout the body. In the lungs, each hemoglobin molecule can bind up to four oxygen molecules. Cells that require oxygen for energy production and metabolic processes then receive this oxygen.
In addition to transporting oxygen, hemoglobin also critically transports carbon dioxide from tissues back to the lungs. Carbon dioxide, a metabolic waste product, binds to hemoglobin, forming carbaminohemoglobin. The lungs then expel this bound carbon dioxide through exhalation, accounting for about 20-25% of the body’s carbon dioxide.
Hemoglobin helps maintain the pH balance in our blood by buffering changes in acidity. When hemoglobin picks up carbon dioxide, it forms bicarbonate ions, which help neutralize pH variations. This buffering capability is vital for maintaining stable physiological conditions.
It also influences the regulation of blood flow and distribution around the body. By binding with gases like nitric oxide, hemoglobin can help relax the blood vessels, increasing blood flow and improving oxygen delivery to needy areas of the body.
Ideal hemoglobin levels vary: men should have 13.8 to 17.2 grams per deciliter, and women, 12.1 to 15.1 grams per deciliter.
Hemoglobin’s major components are globin proteins and heme groups, essential for oxygen transport.
Hemoglobin binds to oxygen in the lungs and carbon dioxide in tissues, facilitating gas exchange.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary function of hemoglobin in the blood?
Transport nutrients
Carry oxygen
Regulate blood pressure
Fight infections
Which component of hemoglobin binds to oxygen?
Heme
Globin
Platelets
Plasma
Hemoglobin is made up of how many subunits?
Two
Three
Four
Five
What is the iron-containing molecule in hemoglobin called?
Hemoglobin
Myoglobin
Heme
Chlorophyll
What condition is characterized by a deficiency of hemoglobin?
Diabetes
Anemia
Hypertension
Asthma
What is the difference between adult hemoglobin (HbA) and fetal hemoglobin (HbF)?
HbF has more heme groups
HbA is found in adults, HbF is found in fetuses
HbF binds less oxygen
HbA binds more carbon dioxide
In which part of the body is hemoglobin primarily produced?
Liver
Liver
Bone marrow
Lungs
Which test measures the amount of hemoglobin in the blood?
Blood glucose test
Hemoglobin A1c test
Complete blood count (CBC)
Lipid profile
What happens to hemoglobin when it binds to carbon dioxide?
It forms carboxyhemoglobin
It releases oxygen
It converts to myoglobin
It forms deoxyhemoglobin
Which of the following diseases is caused by a mutation in the hemoglobin gene?
Sickle cell disease
Cystic fibrosis
Hemophilia
Thalassemia
Before you leave, take our quick quiz to enhance your learning!

