What is the primary role of potassium in the human body?
Bone formation
Blood clotting
Nerve function
Muscle contraction
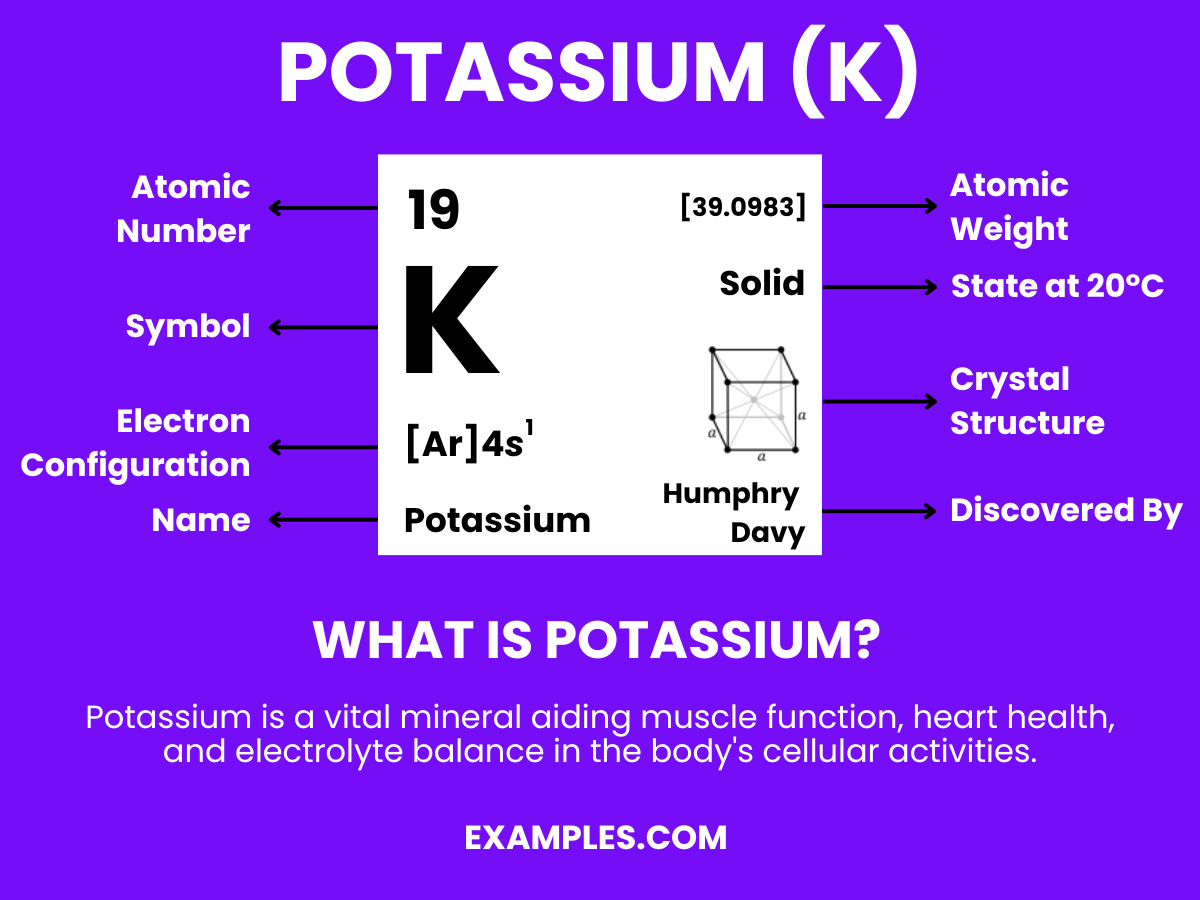
Potassium, an essential nutrient and a key alkali metal, plays a significant role in both biological processes and numerous industrial applications. This guide provides teachers with a thorough understanding of potassium’s properties, its importance in the human body, and its diverse uses in everyday life and technology. Enriched with examples, it’s designed to assist educators in making the concept of potassium engaging and accessible to students, enhancing their learning experience.
Potassium is a soft, silvery-white metal, classified as an alkali metal. It’s highly reactive, especially with water, and is essential for various biological functions in plants and animals, including humans. In nature, potassium is never found in its elemental form due to its high reactivity, but rather in compounds such as potassium chloride. Understanding its properties and uses is crucial in both the field of chemistry and in everyday life applications.
| Lithium |
| Sodium |
| Rubidium |
| Cesium |
| Francium |
Formula: K
Composition: A single potassium atom.
Bond Type: Highly reactive, especially with water.
Molecular Structure: Soft metal.
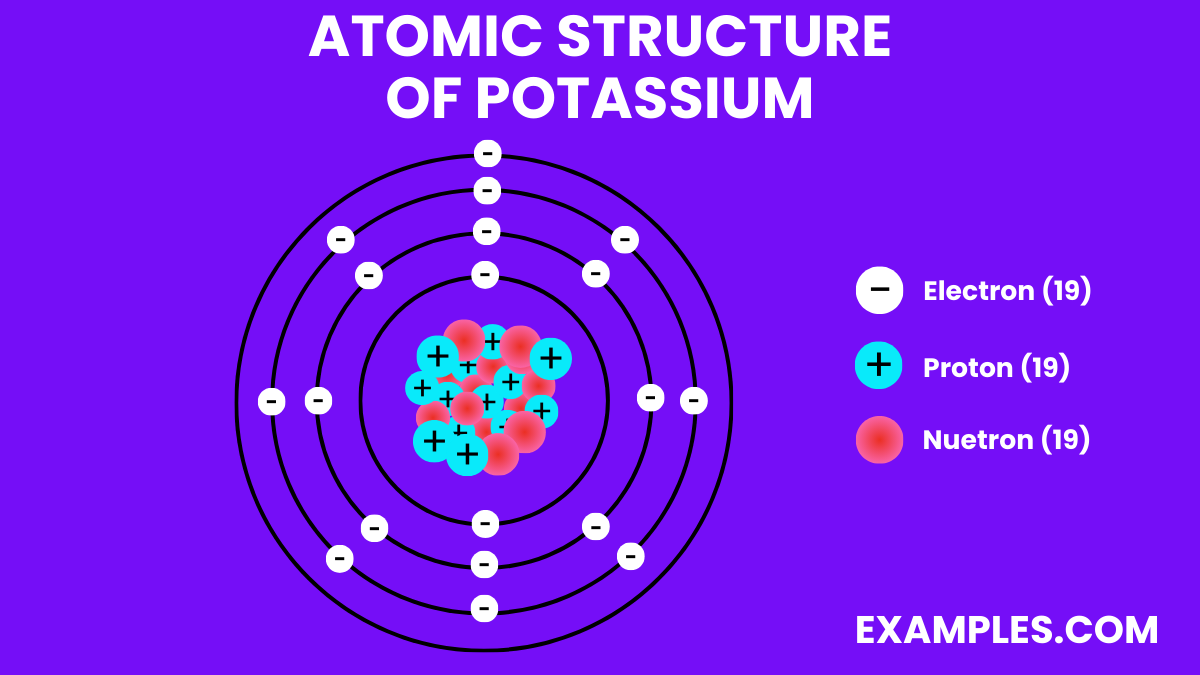
Electron Configuration: 19 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
Significance: Crucial for agriculture as a fertilizer component.
Role in Chemistry: Forms compounds like potassium nitrate (KNO₃), important in agriculture.
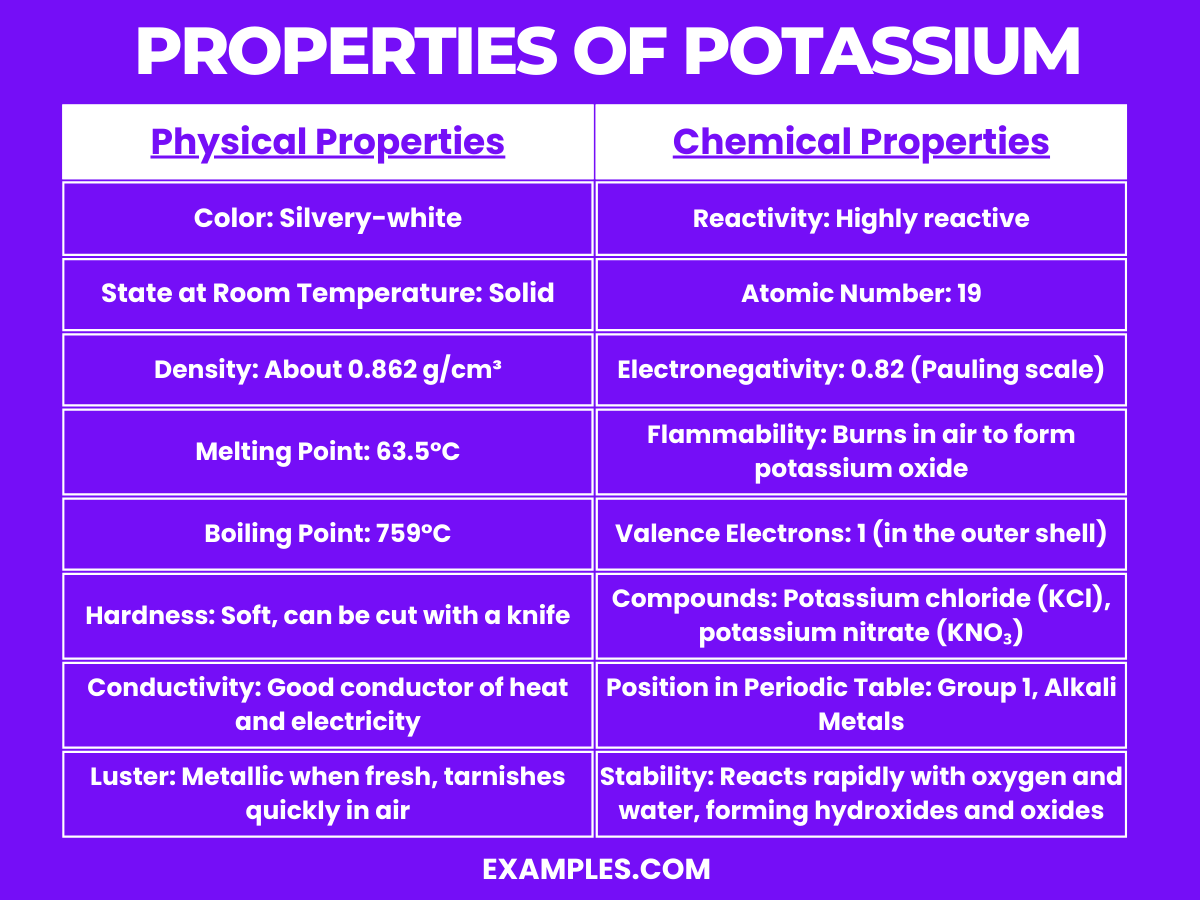
| Physical Property | Description |
|---|---|
| Color | Silvery-white |
| State at Room Temperature | Solid |
| Density | About 0.862 g/cm³ |
| Melting Point | 63.5°C (146.3°F) |
| Boiling Point | 759°C (1398°F) |
| Hardness | Soft, can be cut with a knife |
| Conductivity | Good conductor of heat and electricity |
| Luster | Metallic when fresh; tarnishes quickly in air due to oxidation |
Potassium, a member of the alkali metals, exhibits several distinctive chemical properties:
| Property | Value with Unit |
|---|---|
| Boiling Point | 759 °C |
| Melting Point | 63.5 °C |
| Critical Temperature | NA |
| Critical Pressure | NA |
| Heat of Vaporization | 76.9 kJ/mol |
| Heat of Fusion | 2.33 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.757 J/g·K |
| Thermal Conductivity | 102.5 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 856 kg/m³ |
| Viscosity (at 63.5°C) | NA |
| Solubility in Water | Reacts vigorously with water |
| Color | Silvery |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 7.2 µΩ·m |
| Thermal Conductivity | 102.5 W/m·K |
| Electronegativity (Pauling scale) | 0.82 |
| Ionization Energy | 4.3407 eV |
| Property | Value with Unit |
|---|---|
| Atomic Number | 19 |
| Atomic Mass | 39.0983 amu |
| Isotopes | ^39K (93.2581%), ^40K (0.0117%, radioactive), ^41K (6.7302%) |
| Nuclear Spin (for ^39K) | 3/2 ℏ |
| Nuclear Spin (for ^40K) | 4 ℏ |
| Nuclear Spin (for ^41K) | 3/2 ℏ |
| Neutron Cross Section (for ^39K) | 2.1 barns |
| Neutron Cross Section (for ^41K) | 1.46 barns |
| Nuclear Magnetic Moment (for ^39K) | 0.391 µN |
| Nuclear Magnetic Moment (for ^41K) | 0.214 µN |
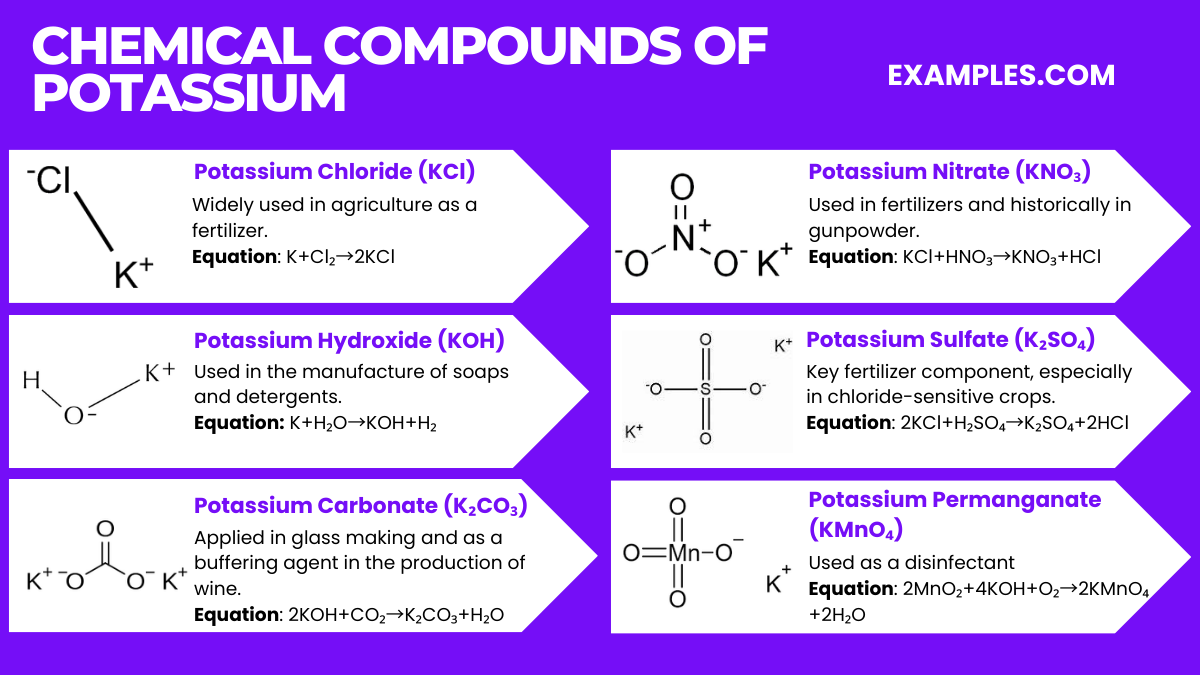
Potassium forms various compounds, each with unique applications. Here are six significant potassium compounds along with their chemical equations:
Potassium has several isotopes, each with specific properties. The table below provides an overview:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| K-39 | 39 | 93.2581 | Stable | – |
| K-40 | 40 | 0.0117 | 1.248 x 10⁹ years | Beta decay, Electron capture |
| K-41 | 41 | 6.7302 | Stable | – |
Potassium is a versatile element with several key applications:
The commercial production of potassium primarily involves the extraction of potassium salts from underground ore deposits and evaporite lakes:
Potassium, an essential mineral, plays several critical roles in human health:
The environmental impact of potassium is generally minimal compared to other elements:
Potassium regulates heart rhythm, muscle function, nerve signals, and fluid balance, and is vital for maintaining cardiovascular health and bone strength.
Low potassium levels, or hypokalemia, can cause muscle weakness, cramps, irregular heartbeats, fatigue, and in severe cases, life-threatening heart problems.
Excessively high potassium levels, known as hyperkalemia, can lead to dangerous heart rhythm disturbances, muscle weakness, and in extreme cases, cardiac arrest.
‘K’ is used for potassium based on its Latin name ‘Kalium’, reflecting historical naming conventions from the time of elemental discovery and classification.
Potassium is a crucial mineral, indispensable for numerous physiological functions in the human body. Its balanced levels are key to maintaining heart health, muscle function, and overall well-being. Understanding the importance of potassium, its health implications, and dietary sources is essential for educators to effectively convey its significance, promoting healthy lifestyle choices and awareness about this vital nutrient.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary role of potassium in the human body?
Bone formation
Blood clotting
Nerve function
Muscle contraction
Which food is a rich source of potassium?
Beef
Rice
Bananas
Bread
What is a common effect of potassium deficiency?
Hypertension
Increased cholesterol
Muscle cramps
Insomnia
How does potassium benefit plant health?
Enhances flower color
Increases seed production
Improves root growth
Helps in water retention
Which industrial process uses potassium?
Steel manufacturing
Glass production
Fertilizer creation
Textile dyeing
What is the effect of high potassium intake on heart health?
Reduces heart rate
Increases blood pressure
Lowers blood pressure
Causes arrhythmia
In what form is potassium most commonly found in nature?
Elemental potassium
Potassium salts
Potassium gases
Potassium liquids
What is a significant risk associated with high levels of potassium?
Anemia
Hyperkalemia
Osteoporosis
Hyperglycemia
How does potassium affect the body’s fluid balance?
Decreases water absorption
Increases dehydration
Regulates fluid levels
Has no effect
Which organ is primarily responsible for regulating potassium levels in the body?
Liver
Heart
Kidneys
Lungs
Before you leave, take our quick quiz to enhance your learning!

