Rubidium is classified under which group in the periodic table?
Alkali metals
Alkaline earth metals
Transition metals
Halogens
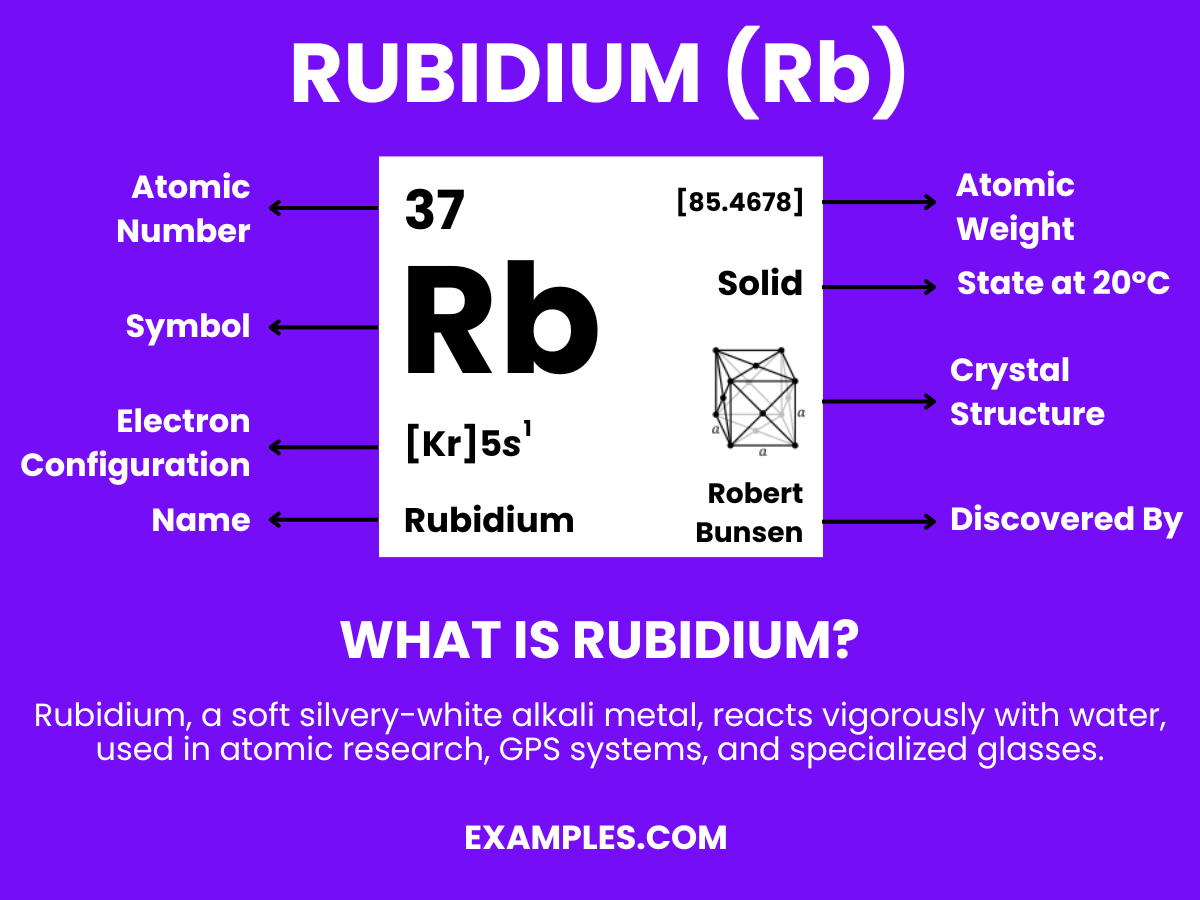
Rubidium might not be a household name, but it plays a significant role in the world of science. As teachers, understanding Rubidium’s unique properties and uses can enrich your chemistry lessons. This guide delves into the essence of Rubidium, offering clear examples and practical tips for its application in educational settings. Whether you’re introducing basic elements or exploring more complex chemical interactions, Rubidium’s fascinating characteristics are sure to captivate and educate.
This soft, silvery-white metallic element is a member of the alkali metal group, found in the periodic table under the symbol ‘Rb’. With an atomic number of 37, Rubidium is known for its high reactivity and is rarely found in its pure form in nature. In simple terms, it’s a fascinating element that reacts vividly with water and has various applications in electronics, research, and even medicine. Understanding Rubidium can offer students a deeper insight into chemical properties and the exciting world of elements.
| Lithium |
| Sodium |
| Potassium |
| Cesium |
| Francium |

| Property | Description |
|---|---|
| Appearance | Soft, silvery-white metal. |
| Atomic Number | 37 |
| Density | Approximately 1.532 g/cm³ at 20°C. |
| Melting Point | 39.31°C |
| Boiling Point | 688°C |
| State at Room Temperature | Solid |
| Conductivity | Good conductor of electricity. |
| Malleability | Highly malleable and ductile. |
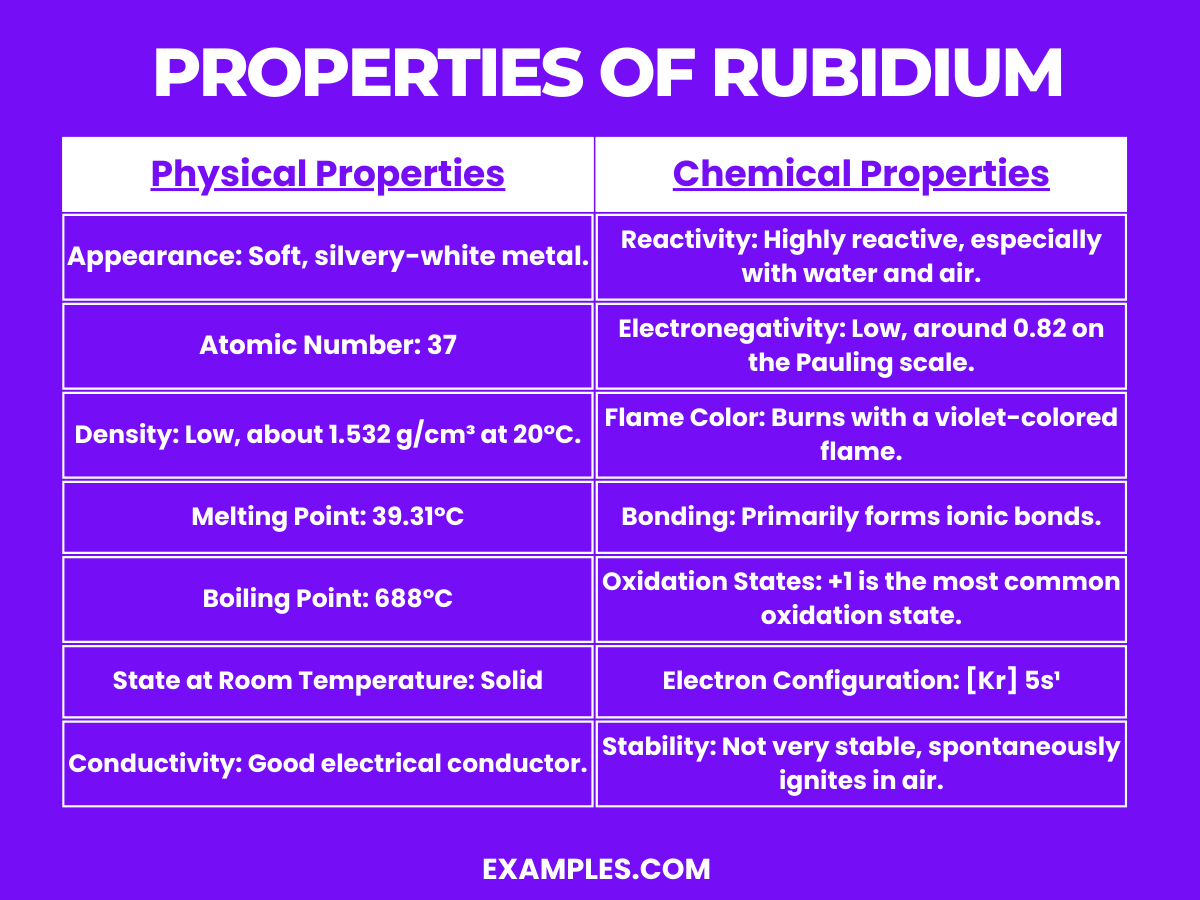
Rubidium is a highly reactive alkali metal, with interesting chemical properties:
These chemical properties make rubidium an element of great interest in chemistry, particularly in the study of alkali metals and their reactions.
| Property | Value with Unit |
|---|---|
| Boiling Point | 688 °C |
| Melting Point | 39.31 °C |
| Critical Temperature | Not Available |
| Critical Pressure | Not Available |
| Heat of Vaporization | 75.77 kJ/mol |
| Heat of Fusion | 2.19 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.363 J/g·K |
| Thermal Conductivity | 58.2 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 1.532 g/cm³ |
| Viscosity (at melting point) | Not Available |
| Solubility | Reacts with water, soluble in liquid ammonia |
| Color | Silvery-white |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 12.5 nΩ·m |
| Thermal Conductivity | 58.2 W/m·K |
| Magnetic Susceptibility | +0.00017 cm³/mol |
| Electronegativity (Pauling scale) | 0.82 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 37 |
| Atomic Mass | 85.4678 amu (Natural abundance average) |
| Isotopes | ^85Rb (72.17%), ^87Rb (27.83%) |
| Nuclear Spin (for ^85Rb) | 5/2 ℏ |
| Nuclear Spin (for ^87Rb) | 3/2 ℏ |
| Neutron Cross Section (for ^85Rb) | 0.48 barns |
| Neutron Cross Section (for ^87Rb) | 12.8 barns |
| Nuclear Magnetic Moment (for ^85Rb) | 1.353 µN |
| Nuclear Magnetic Moment (for ^87Rb) | 2.751 µN |
| Isotope | Natural Abundance | Half-Life | Decay Mode | Applications |
|---|---|---|---|---|
| Rubidium-85 | 72.17% | Stable | Stable (non-radioactive) | Common form of rubidium, used in research and industry. |
| Rubidium-87 | 27.83% | 4.9 × 10¹⁰ years | Beta decay to strontium-87 | Used in radiometric dating and research. |
| Rubidium-86 | Trace | 18.65 days | Beta decay to strontium-86 | Used in nuclear medicine for imaging and diagnosis. |
| Rubidium-84 | Trace | 32.9 days | Electron capture to krypton-84 | Used in research, especially in physics. |
| Rubidium-83 | Trace | 86.2 days | Electron capture to krypton-83 | Used in medical and scientific research. |
| Rubidium-82 | Trace | 1.273 minutes | Beta decay to strontium-82 | Used in nuclear medicine and research. |
Rubidium isotopes, especially rubidium-87, are significant in scientific research due to their long half-life and beta decay process, making them useful in various fields such as geochronology and medical imaging.
The commercial production of rubidium is generally not conducted as a primary mining or manufacturing process due to its relatively limited application and abundance. Instead, rubidium is typically obtained as a byproduct from the processing of other minerals. Here are the key aspects of its production:
Rubidium, like other alkali metals, is not found free in nature and only occurs in compounds. The health effects of rubidium, therefore, largely depend on its compounds’ forms and exposure levels. Here’s a detailed look:
Rubidium’s environmental impact is minimal compared to many other elements, mainly because of its relatively low abundance and limited use in industrial applications. However, some points are worth noting:
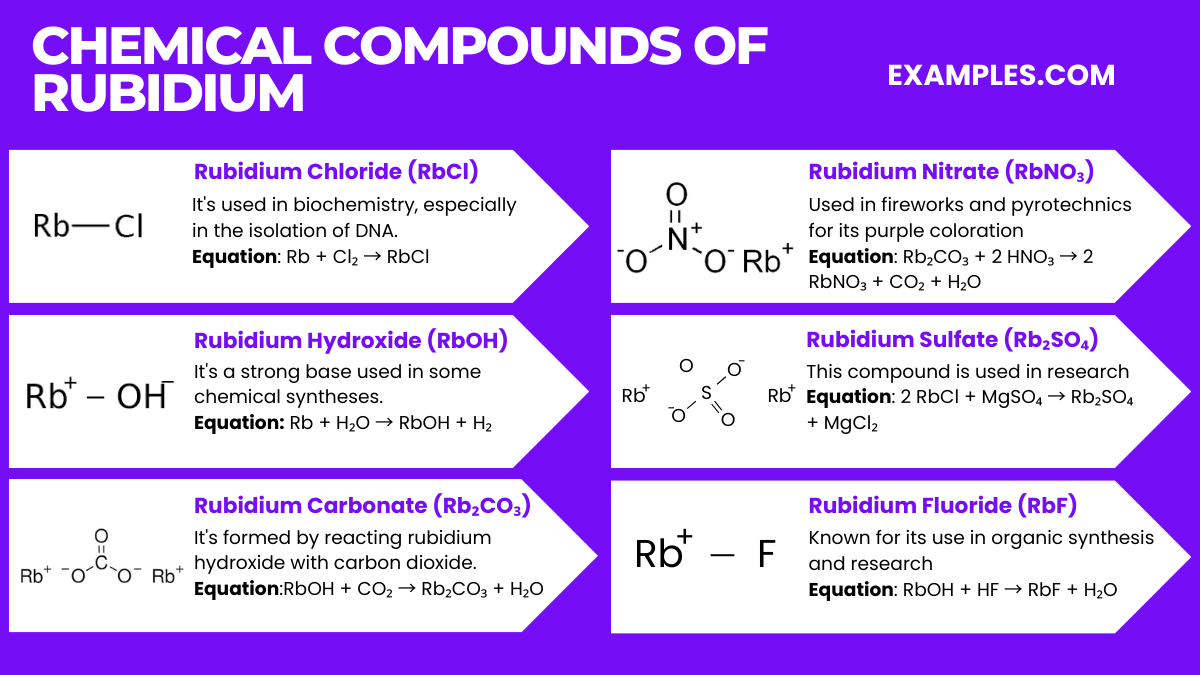
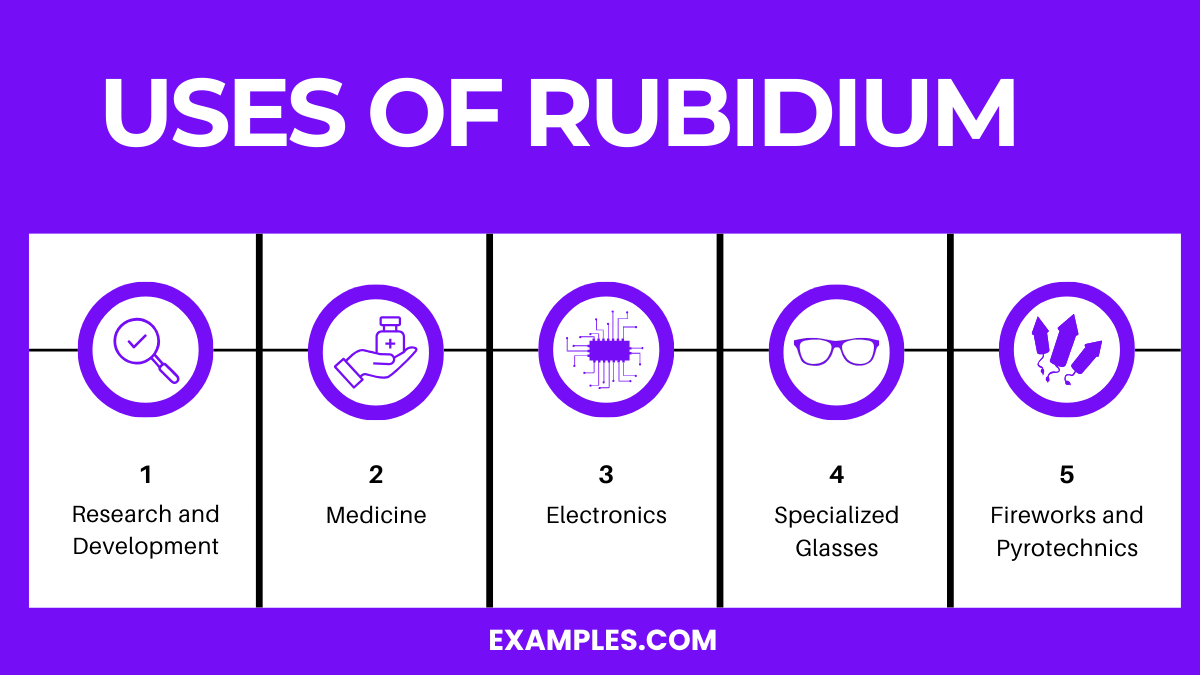
Rubidium is primarily used in electronics, research, and special glasses. Its applications include photoelectric cells and vacuum tubes.
Rubidium is moderately toxic. It reacts violently with water and can cause skin and eye irritation upon contact.
Touching rubidium is unsafe. It reacts aggressively with moisture, including skin moisture, posing burn risks.
Natural rubidium is not significantly radioactive. However, its isotope Rubidium-87 is mildly radioactive but generally not hazardous.
Rubidium has no known biological role in the human body and is not considered essential for life.
Rubidium is a soft, silvery-white alkali metal, known for its high reactivity and low melting point.
Rubidium, a highly reactive alkali metal, has intriguing applications in electronics and scientific research. While not biologically essential, its properties demand careful handling due to its reactivity and mild toxicity. This guide underscores rubidium’s unique role in modern technology and science, offering insights and tips for safe and informed usage in various fields.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Rubidium is classified under which group in the periodic table?
Alkali metals
Alkaline earth metals
Transition metals
Halogens
What is the atomic number of rubidium?
35
37
40
45
Rubidium has a melting point of approximately:
39°C
70°C
102°C
145°C
Rubidium is found naturally in which of the following minerals?
Hematite
Lepidolite
Bauxite
Fluorite
What is the primary application of rubidium in modern technology?
As a fuel in nuclear reactors
In the manufacture of glass
In atomic clocks
In the construction of batteries
What is the symbol for rubidium in the periodic table?
Rh
Rb
Ru
Ra
Rubidium reacts vigorously with which substance?
Oxygen
Hydrogen
Water
Nitrogen
What color does rubidium emit when exposed to a flame?
Green
Blue
Red-violet
Yellow
In terms of abundance, rubidium is classified as:
Rare
Abundant
Moderately abundant
Extremely rare
Which isotope of rubidium is primarily used in radiometric dating?
Rb-87
Rb-85
Rb-86
Rb-88
Before you leave, take our quick quiz to enhance your learning!

