What is the atomic number of Sodium?
10
11
12
13
Sodium, an essential alkali metal, plays a pivotal role in both the scientific world and our daily lives. This comprehensive guide delves into the fundamental aspects of sodium, exploring its properties, diverse applications, and critical safety tips. Perfect for teachers, it provides practical examples to enrich classroom discussions, from its role in the human body to its use in industries. Understanding sodium’s dynamics enhances educational experiences, making science both relatable and intriguing.
Sodium is a highly reactive, silvery-white alkali metal, known for its abundance in the Earth’s crust and oceans. It’s essential for various biological functions and is widely used in compounds like sodium chloride (table salt). In its pure form, sodium reacts swiftly with water and air, necessitating careful handling. Its properties and compounds make it a fundamental element in both chemistry education and various industrial applications.
| Lithium |
| Potassium |
| Rubidium |
| Cesium |
| Francium |
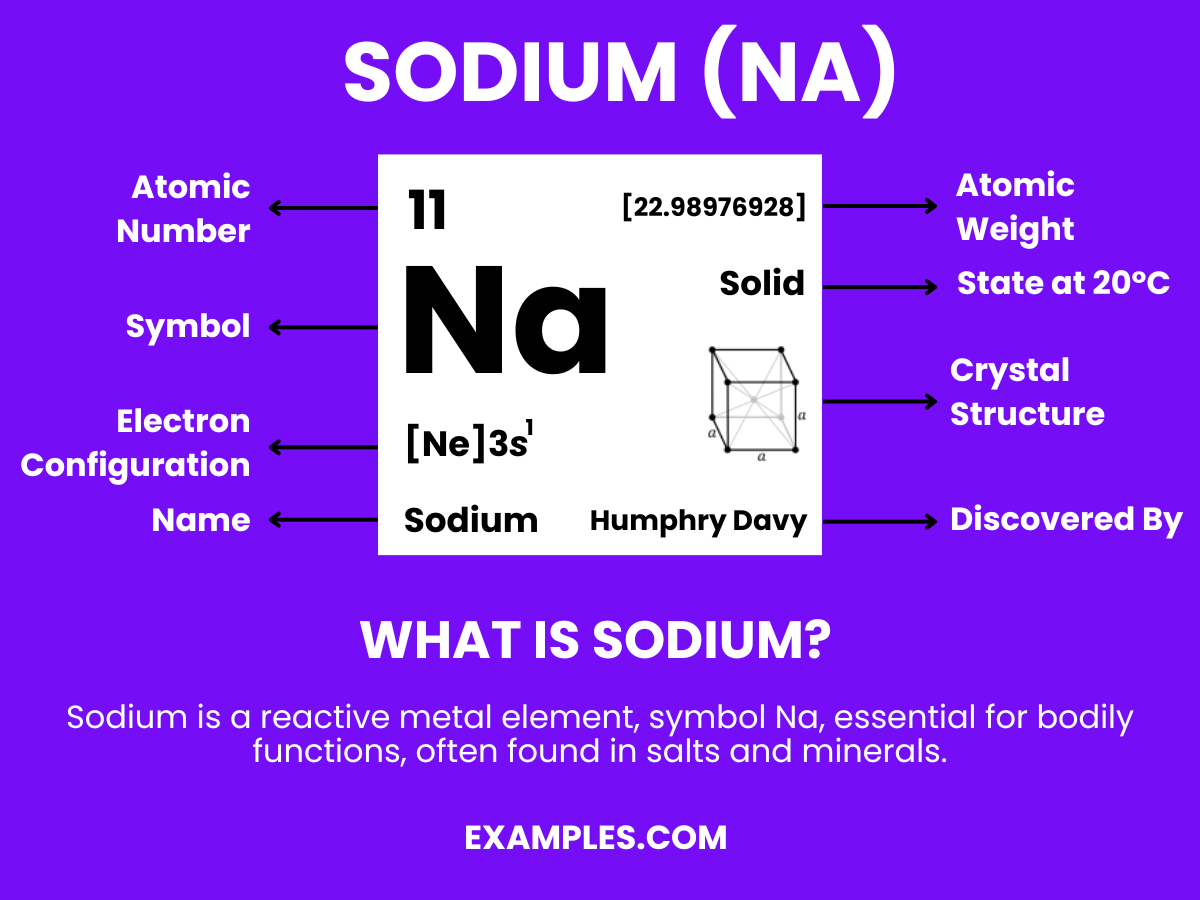
Formula: Na
Composition: A single sodium atom.
Bond Type: Highly reactive, especially with water.
Molecular Structure: Soft metal.
Electron Configuration: 11 electrons; configuration 1s² 2s² 2p⁶ 3s¹.
Significance: Essential in daily life, used in table salt and industrial applications.
Role in Chemistry: Forms vital compounds like sodium chloride (NaCl).
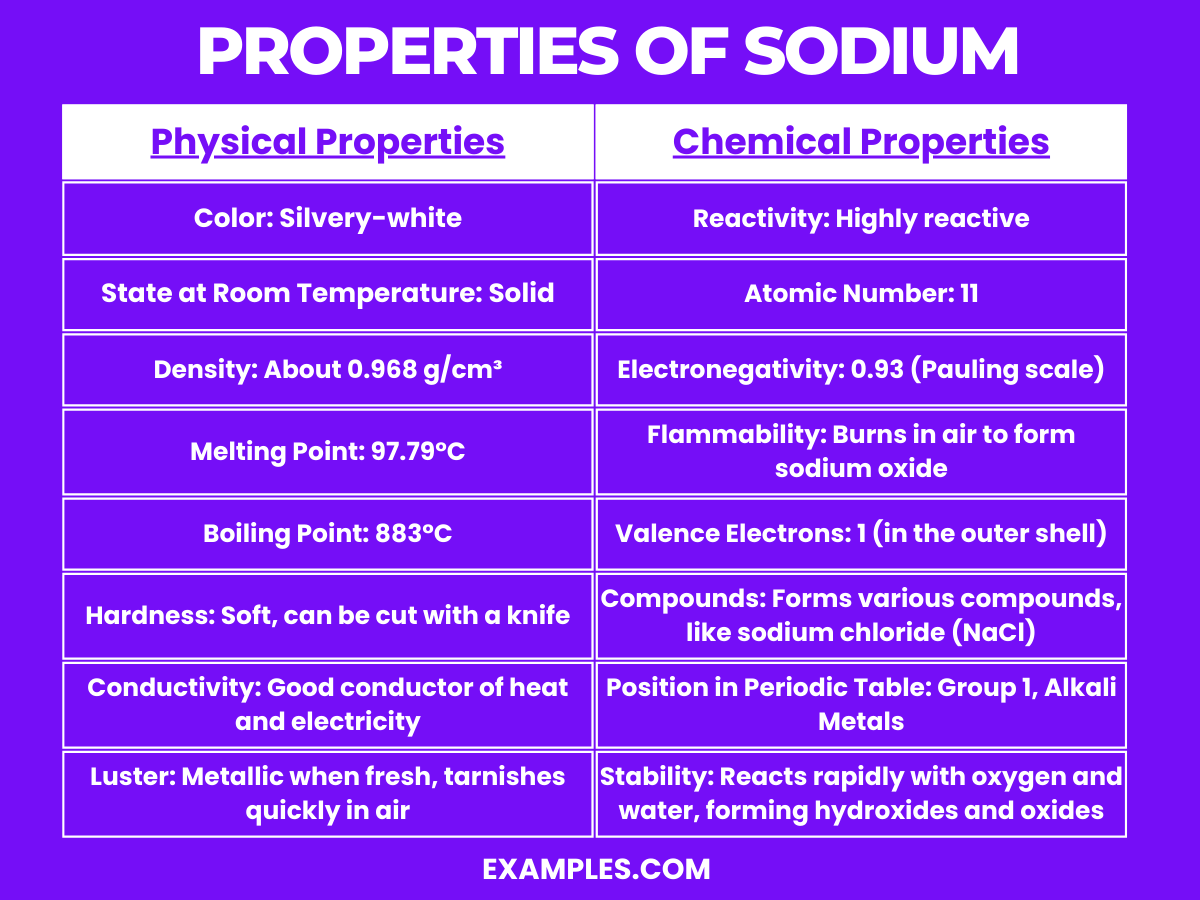
| Physical Property | Description |
|---|---|
| Color | Silvery-white |
| State at Room Temperature | Solid |
| Density | About 0.968 g/cm³ |
| Melting Point | 97.79°C (208°F) |
| Boiling Point | 883°C (1621°F) |
| Hardness | Soft, can be cut with a knife |
| Conductivity | Good conductor of heat and electricity |
| Luster | Metallic when fresh; tarnishes quickly in air due to oxidation |
Sodium exhibits several notable chemical properties:
| Property | Value with Unit |
|---|---|
| Boiling Point | 883 °C |
| Melting Point | 97.79 °C |
| Critical Temperature | 2573 °C |
| Critical Pressure | 35 MPa |
| Heat of Vaporization | 97.42 kJ/mol |
| Heat of Fusion | 2.60 kJ/mol |
| Specific Heat Capacity (at 25°C) | 1.23 J/g·K |
| Thermal Conductivity | 142 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 968 kg/m³ |
| Viscosity (at 100°C) | 0.71 mPa·s |
| Solubility in Water | Reacts with water |
| Refractive Index | NA (Metallic) |
| Surface Tension (at melting point) | 180 mN/m |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 4.7 µΩ·m |
| Thermal Conductivity | 142 W/m·K |
| Magnetic Susceptibility | -5 × 10^-6 cm^3/mol |
| Electronegativity (Pauling scale) | 0.93 |
| Property | Value with Unit |
|---|---|
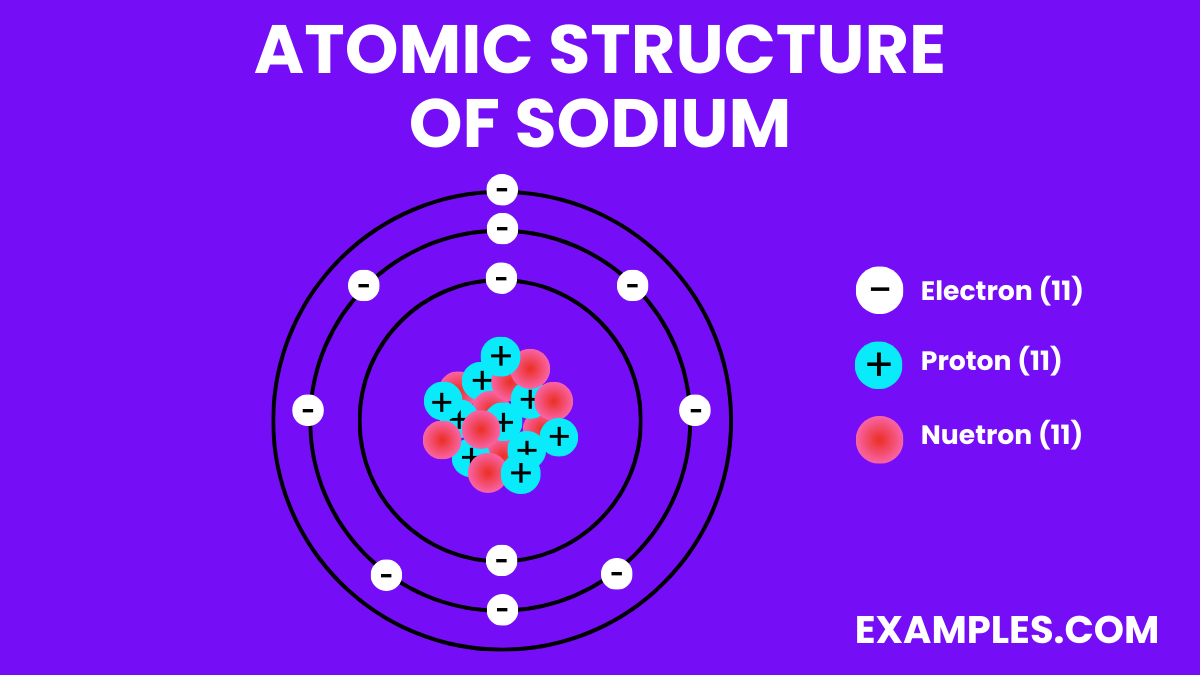
| Atomic Number | 11 |
| Atomic Mass | 22.98976928 amu |
| Isotopes | ^23Na (100%) |
| Nuclear Spin (for ^23Na) | 3/2 ℏ |
| Neutron Cross Section (for ^23Na) | 0.53 barns |
| Nuclear Magnetic Moment (for ^23Na) | 2.2175 µN |
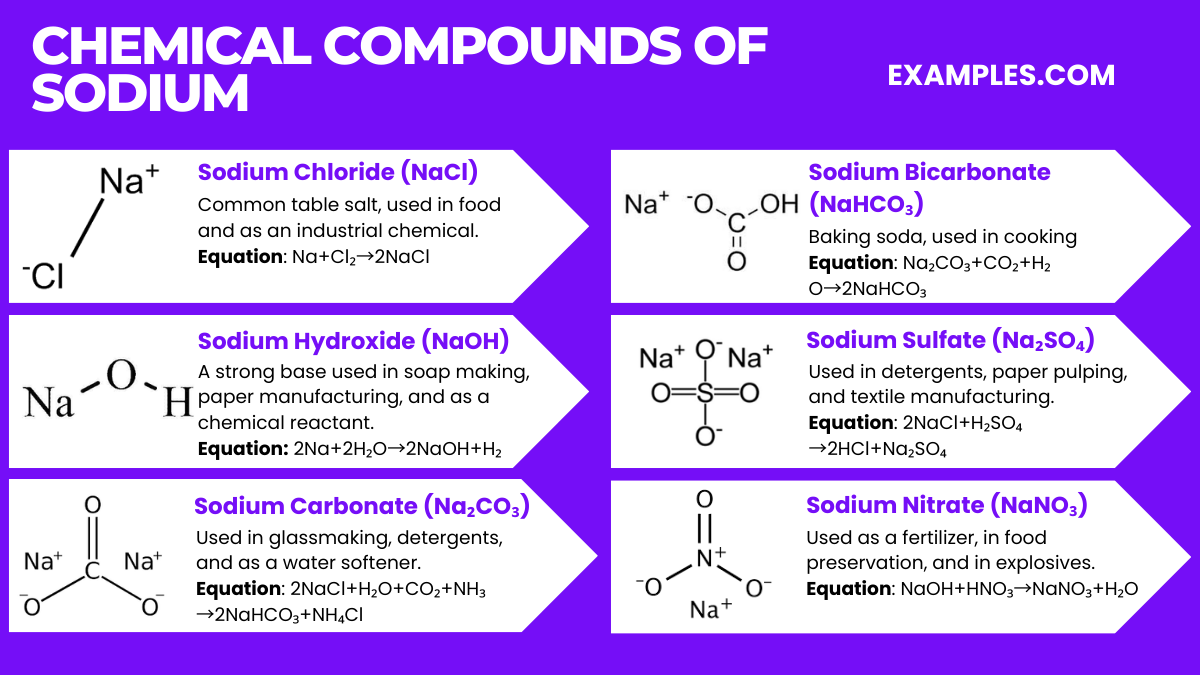
Sodium forms a range of important chemical compounds, each with distinct applications. Here are six key sodium compounds along with their chemical equations:
Sodium has several isotopes, each with specific characteristics. The table below provides an overview:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| Na-22 | 22 | Synthetic | 2.6 years | Beta decay |
| Na-23 | 23 | 100 | Stable | – |
| Na-24 | 24 | Synthetic | 15 hours | Beta decay |
The most common and naturally occurring isotope is Sodium-23, which constitutes all naturally occurring sodium. The other isotopes, like Sodium-22 and Sodium-24, are artificially produced and have applications in medical and scientific research, particularly in nuclear medicine and as tracers.
Sodium has various applications due to its chemical properties. Here are five of its most significant uses:
Sodium is primarily produced through the electrolysis of sodium chloride (table salt), a process known as the Downs process:
Sodium, an essential nutrient, has significant impacts on human health, both beneficial and potentially harmful:
The environmental impact of sodium is primarily related to its compounds and industrial usage:
Sodium regulates blood pressure and fluid balance, supports nerve function and muscle contraction, but excessive intake can lead to health issues like hypertension.
Sodium is a soft, silvery-white metal, highly reactive and a key component of table salt (sodium chloride) when combined with chlorine.
Sodium is used in table salt, industrial chemicals (like caustic soda), street lighting (sodium vapor lamps), and as a heat conductor in some nuclear reactors.
Symptoms of excessive sodium intake include high blood pressure, swelling or edema, headache, dehydration, and in severe cases, heart and kidney problems.
Sodium, a vital element in both biology and industry, plays key roles ranging from regulating bodily functions to industrial applications. However, its management, both in dietary intake and environmental impact, is crucial. Understanding sodium’s properties, uses, and health implications empowers educators to teach its significance effectively, emphasizing the importance of balance in its consumption and usage.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Sodium?
10
11
12
13
Which of the following properties is NOT characteristic of sodium?
Silver-colored
Highly reactive
Heavy and dense
Soft enough to cut with a knife
Sodium is essential in the human body primarily for:
Bone formation
Nerve impulse transmission
Muscle contraction
Both B and C
What compound does sodium form when it reacts with water?
Sodium hydroxide
Sodium chloride
Sodium oxide
Sodium peroxide
Which industry primarily uses sodium in large amounts?
Textile
Paper
Glass making
Petroleum
The natural source of sodium is primarily:
Fresh water
Rocks and minerals
Plant materials
Atmospheric gases
Sodium vapor lamps emit light that is primarily:
Red
Blue
Yellow
Green
A common dietary source of sodium is:
Fresh fruits
Red meat
Table salt
Whole grains
Sodium's role in food preservation primarily involves:
Enhancing flavor
Absorbing moisture
Preventing microbial growth
All of the above
In terms of reactivity, sodium:
Does not react with oxygen
Reacts violently with water
Is stable at high temperatures
Cannot form compounds with non-metals
Before you leave, take our quick quiz to enhance your learning!

