What is the atomic number of Francium?
87
88
89
90
Francium, a rare and intriguing element, captures the curiosity of educators and students alike. This comprehensive guide delves into the heart of Francium, exploring its unique properties and applications. Ideal for teaching in classrooms or enhancing scientific understanding, we unravel the mysteries of this elusive element, offering clear explanations and practical examples. Our focus on Francium’s role in modern science and education makes this guide a valuable resource for those eager to expand their knowledge in the fascinating world of chemistry.
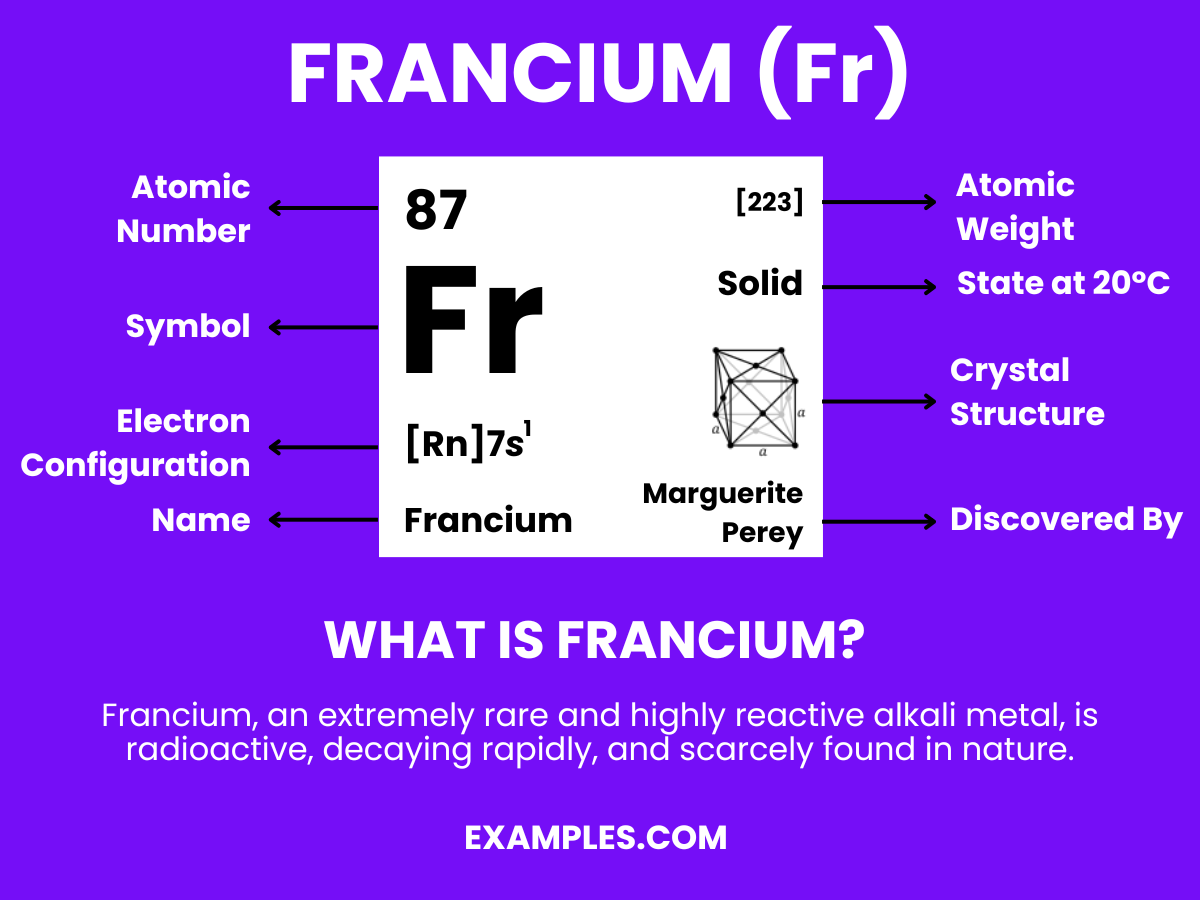
Francium is a chemical element with significant scientific intrigue, primarily due to its rarity and position in the periodic table. As the heaviest known alkali metal, Francium exhibits unique properties that captivate chemists and educators. It has a symbol ‘Fr’ and atomic number 87. Due to its extreme reactivity and scarcity, Francium is not found freely in nature and is mostly studied in minute amounts. This elusive element serves as a compelling topic in the field of chemistry, offering rich educational opportunities for teachers to engage students in exploring the nuances of the periodic table and chemical reactions.
| Lithium |
| Sodium |
| Potassium |
| Rubidium |
| Cesium |
| Property | Description |
|---|---|
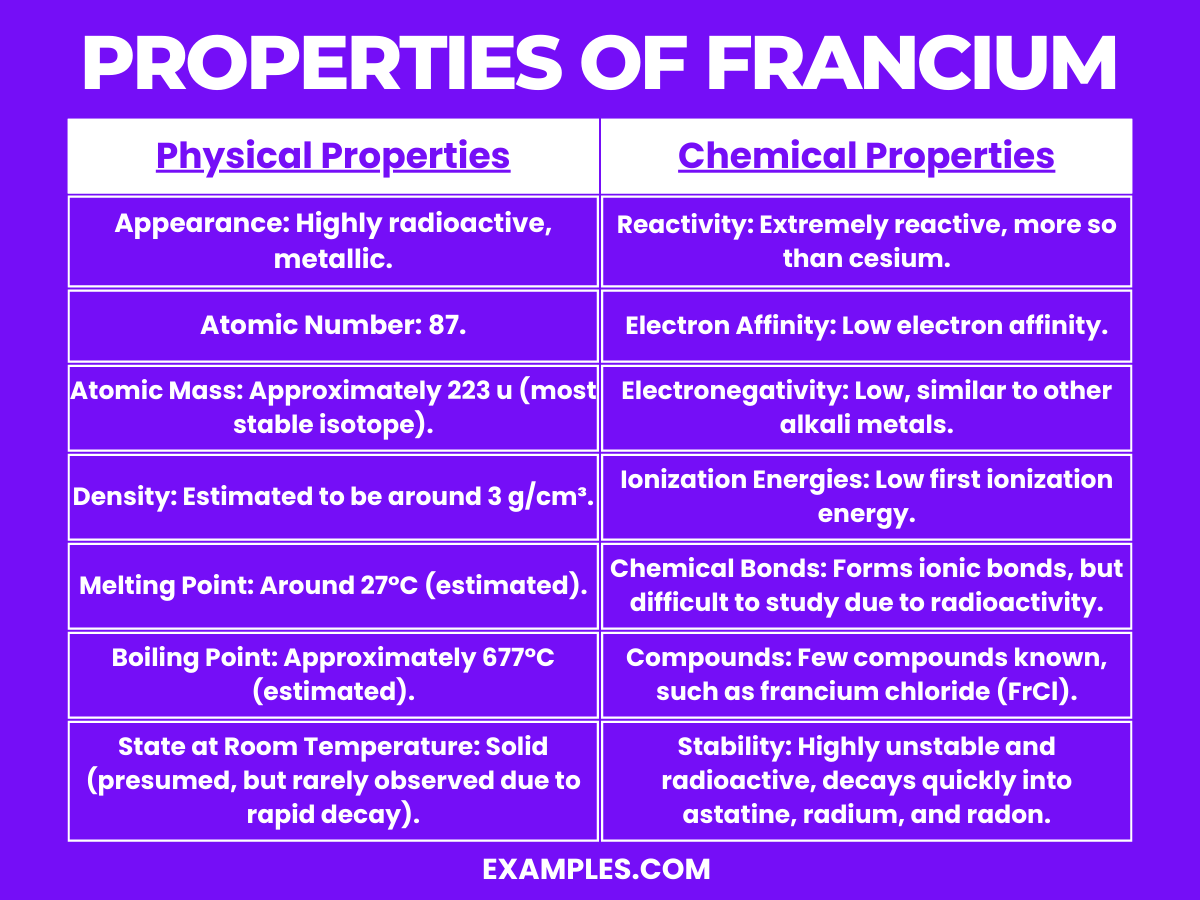
| Appearance | Highly radioactive and metallic (theoretical appearance) |
| Atomic Number | 87 |
| Atomic Mass | Approximately 223 u (most stable isotope) |
| Density | Estimated around 3 g/cm³ (theoretical) |
| Melting Point | Around 27°C (estimated) |
| Boiling Point | Approximately 677°C (estimated) |
| State | Solid at room temperature (presumed, rarely observed) |
| Radioactivity | Extremely radioactive, decays rapidly |
Francium, being one of the least stable and most elusive elements, has chemical properties that are largely theoretical or derived from the behavior of other alkali metals. Here are some of its key chemical properties:
| Property | Value with Unit |
|---|---|
| Boiling Point | Estimated 677 °C (Theoretical) |
| Melting Point | Approx. 27 °C (Theoretical) |
| Critical Temperature | Not available |
| Critical Pressure | Not available |
| Heat of Vaporization | Not well-documented |
| Heat of Fusion | Not well-documented |
| Specific Heat Capacity | Not well-documented |
| Property | Value with Unit |
|---|---|
| Density | Approx. 1.87 g/cm³ (Theoretical) |
| Viscosity | Not available |
| Solubility | Presumed to react violently with water like other alkali metals |
| Phase at Room Temperature | Solid (Assumed) |
| Color | Not well-documented; likely metallic |
| Property | Value with Unit |
|---|---|
| Electrical Conductivity | Expected to be high (Theoretical) |
| Electronegativity (Pauling scale) | Approx. 0.7 (Theoretical) |
| Ionization Energy | Estimated 380 kJ/mol (Theoretical) |
| Electron Affinity | Not well-documented |
| Property | Value with Unit |
|---|---|
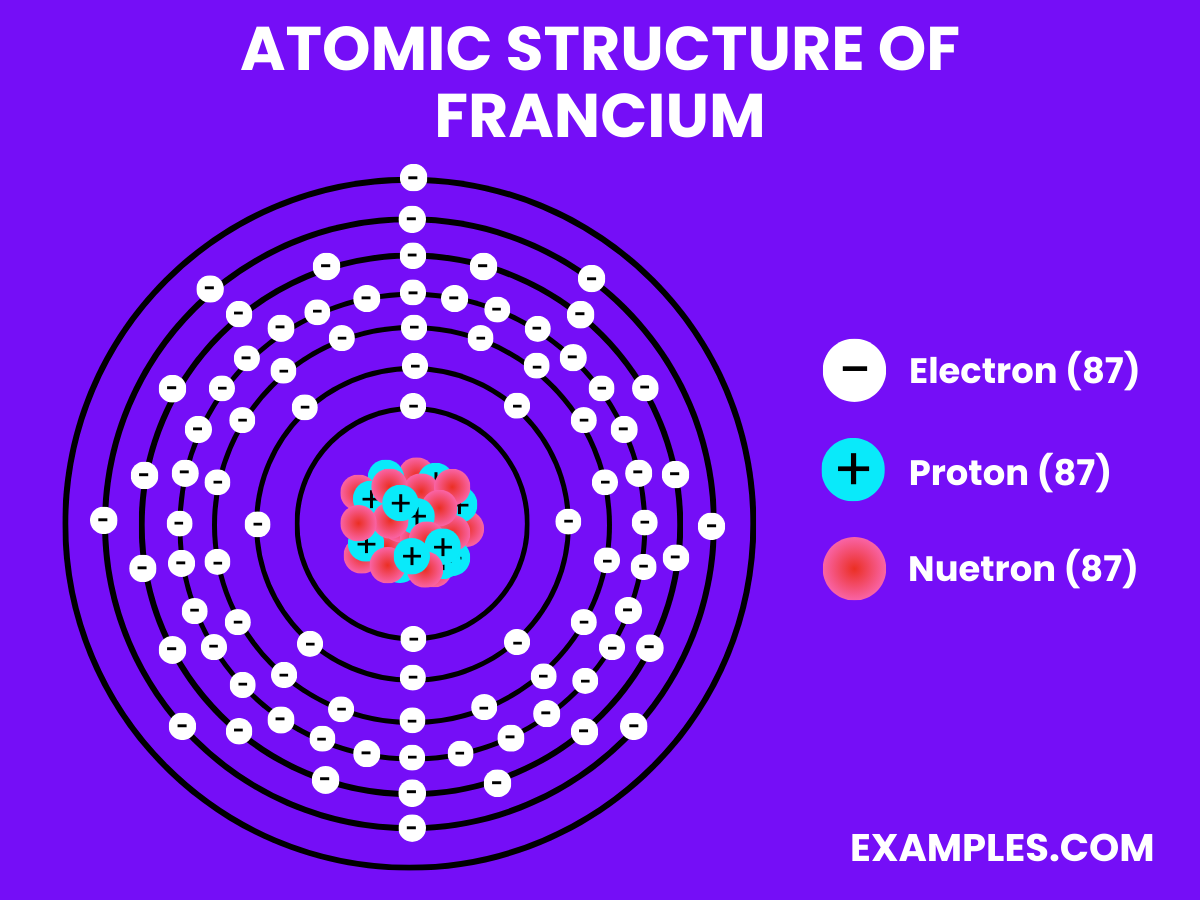
| Atomic Number | 87 |
| Atomic Mass | 223 amu (Most stable isotope) |
| Isotopes | Over 30, all radioactive |
| Half-life of Most Stable Isotope (^223Fr) | 22 minutes |
| Mode of Decay | Beta decay |
| Isotope | Half-life | Mode of Decay | Notes |
|---|---|---|---|
| Francium-212 | 19.5 minutes | Beta decay | Decays to radon-212. |
| Francium-213 | 34.6 seconds | Beta decay | Decays to radon-213. |
| Francium-214 | 5 milliseconds | Alpha decay | One of the shortest-lived isotopes, decays to radon-214. |
| Francium-220 | 27.4 seconds | Beta decay | Decays to radon-220. |
| Francium-221 | 4.8 minutes | Beta decay | Most stable isotope, decays to radium-221. |
| Francium-223 | 22 minutes | Beta decay | Decays to radium-223, often used in scientific research. |
Given its extreme rarity and radioactivity, Francium has very limited practical applications. However, here are five potential or theoretical uses:
Commercial production of Francium is not feasible due to several reasons:
As Francium is highly radioactive and exists only in trace amounts, its health effects have not been directly observed in humans. However, based on its properties, the following can be inferred:
Francium’s environmental effects are largely theoretical due to its rarity and short half-life:
Francium, primarily used in scientific research, offers insights into nuclear physics and atomic structure due to its radioactivity and rarity.
Francium naturally occurs in trace amounts, continuously produced and decayed in uranium and thorium ores, but is not present in large quantities.
Francium is highly radioactive and can cause severe health issues like radiation poisoning if encountered, but its scarcity makes direct human exposure extremely unlikely.
Francium’s rarity is due to its highly unstable nature, with no stable isotopes, leading to rapid decay and transient existence in nature.
At any given time, only a few grams of francium exist on Earth, found in trace amounts due to its continuous radioactive decay.
Francium’s theoretical high cost stems from its extreme rarity and the complex, resource-intensive processes required to isolate even minute quantities.
Francium, a fascinating yet elusive element, captivates the scientific community with its rarity and radioactive properties. Understanding Francium enhances our knowledge of atomic behavior and nuclear physics. However, its practical applications are limited. This guide has explored Francium’s characteristics, emphasizing the importance of theoretical study over practical use, and offering insights into the intriguing world of this rare element.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Francium?
87
88
89
90
Francium belongs to which group in the periodic table?
Alkali metals
Alkaline earth metals
Transition metals
Halogens
Which of the following is a characteristic property of Francium?
High melting point
High reactivity with water
Low electrical conductivity
High stability
Francium is most similar to which of the following elements?
Cesium
Magnesium
Iron
Neon
What is the most common use of Francium?
In fireworks
In nuclear batteries
In research
In construction
What type of decay does Francium primarily undergo?
Alpha decay
Beta decay
Gamma decay
Spontaneous fission
What is the most stable isotope of Francium?
Francium-221
Francium-222
Francium-223
Francium-224
How is Francium typically produced?
Through mining
In nuclear reactors
By electrolysis
As a byproduct in uranium decay
Why is Francium rarely found in nature?
It is artificially produced
It has a very short half-life
It is not chemically active
It is a noble gas
Which element does Francium displace in the reactivity series?
Lithium
Sodium
Potassium
Rubidium
Before you leave, take our quick quiz to enhance your learning!

