What is the atomic number of Sulfur?
14
16
18
20
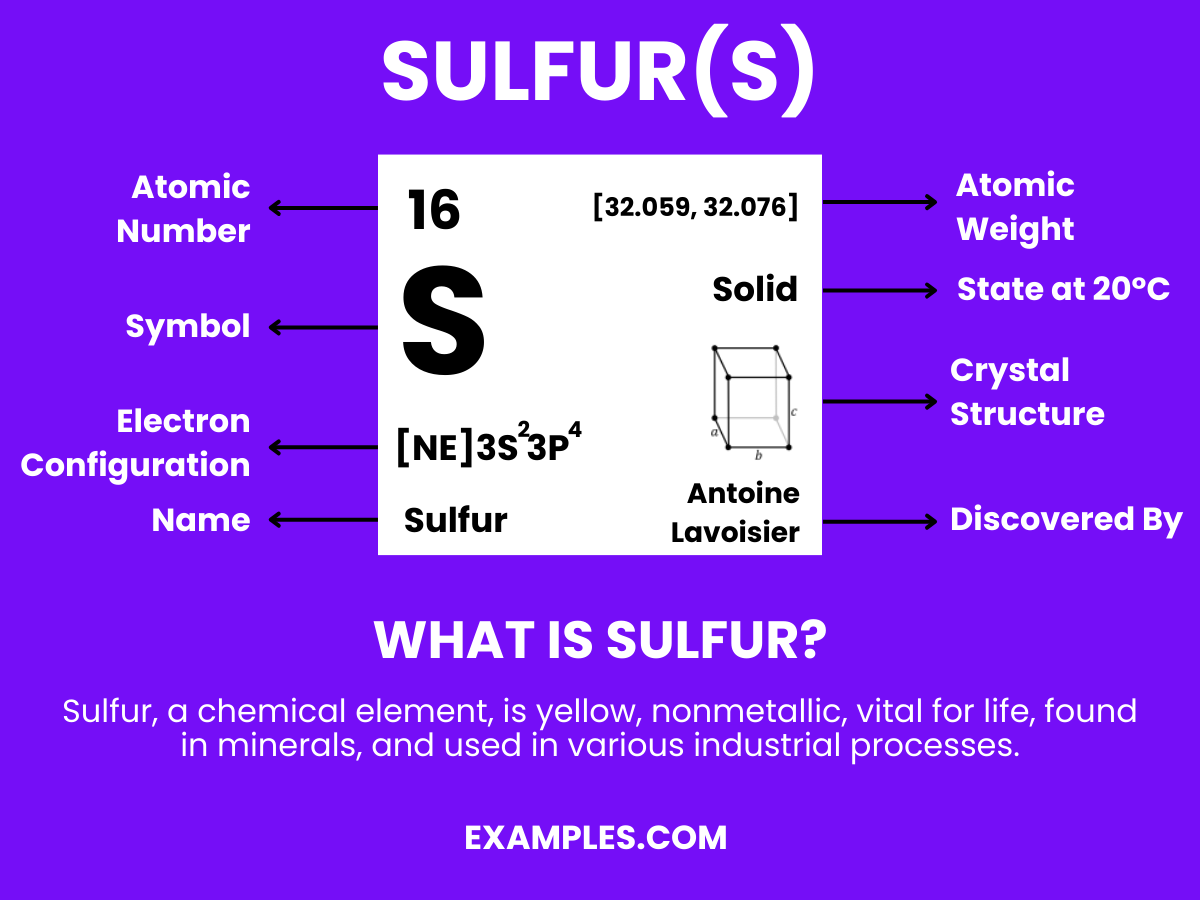
Delve into the multifaceted world of sulfur, a cornerstone element vital for life. This comprehensive guide sheds light on sulfur’s role, from its interaction with hydrogen to its wide-ranging applications in industries and nature. With easy-to-understand examples and practical insights, you’ll navigate the complexities of sulfur like never before. Embrace the journey of discovery and enhance your knowledge with this essential resource.
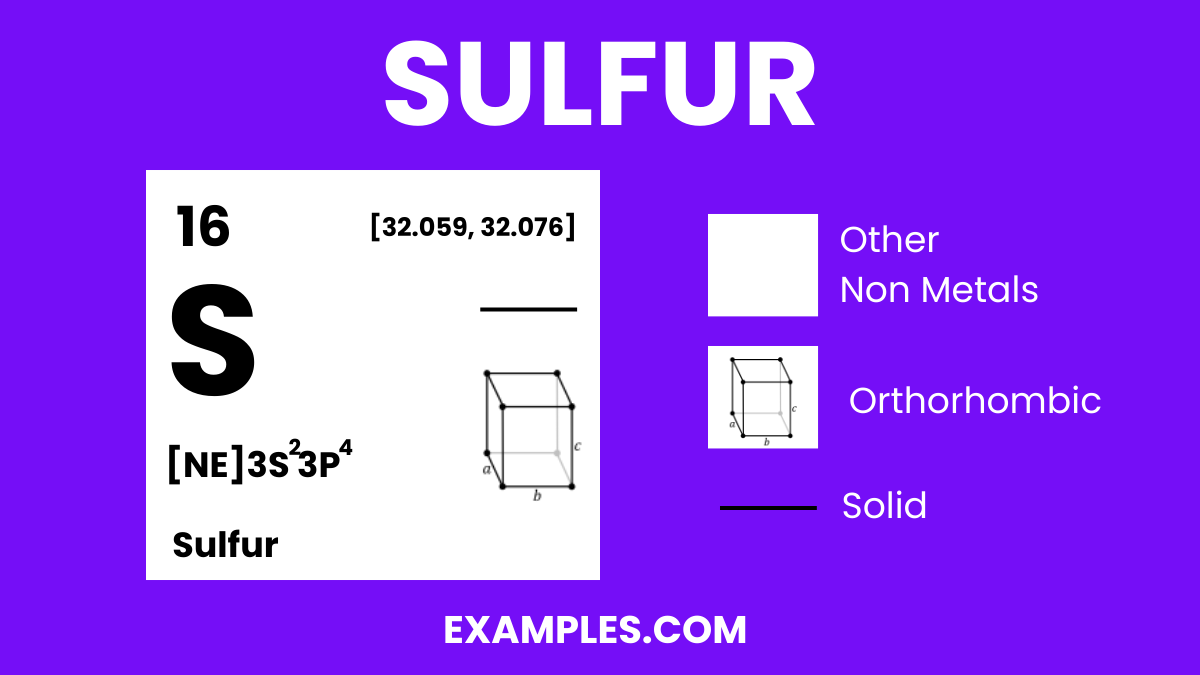
Sulfur, commonly known as the “beauty mineral” due to its pivotal role in collagen synthesis, is a non-metallic element with the atomic number 16. Renowned for its bright yellow crystals and distinct smell, sulfur is essential in various biological and industrial processes. It’s a key component in amino acids, vitamins, and is pivotal in energy metabolism, making it a fundamental element for all living organisms. From gunpowder to fertilizers, sulfur’s versatility extends far and wide, marking its significance in our daily lives.
| Hydrogen | Phosphorus |
| Carbon | Chlorine |
| Nitrogen | Selenium |
| Oxygen | Bromine |
| Fluorine | Iodine |
Formula: S₈
Composition: Eight sulfur atoms.
Bond Type: Weak single bonds forming a crown-shaped ring.
Molecular Structure: Crown-shaped cyclic octatomic molecule.
Electron Configuration: Six valence electrons per atom, forty-eight in total for S₈.
Significance: Crucial for life, involved in amino acid and protein synthesis, and vital for soil fertility.
Role in Chemistry: Used in the manufacture of sulfuric acid, matches, and fireworks. Found in nature as the yellow crystals commonly known as brimstone.

Sulfur, an essential element known for its bright yellow crystals and distinct smell, is a non-metal and the tenth most abundant element in the universe. This guide delves deep into the physical and chemical properties of sulfur, offering educators and students a comprehensive understanding. Let’s explore the multifaceted nature of sulfur in the following table, providing a clear and detailed breakdown.
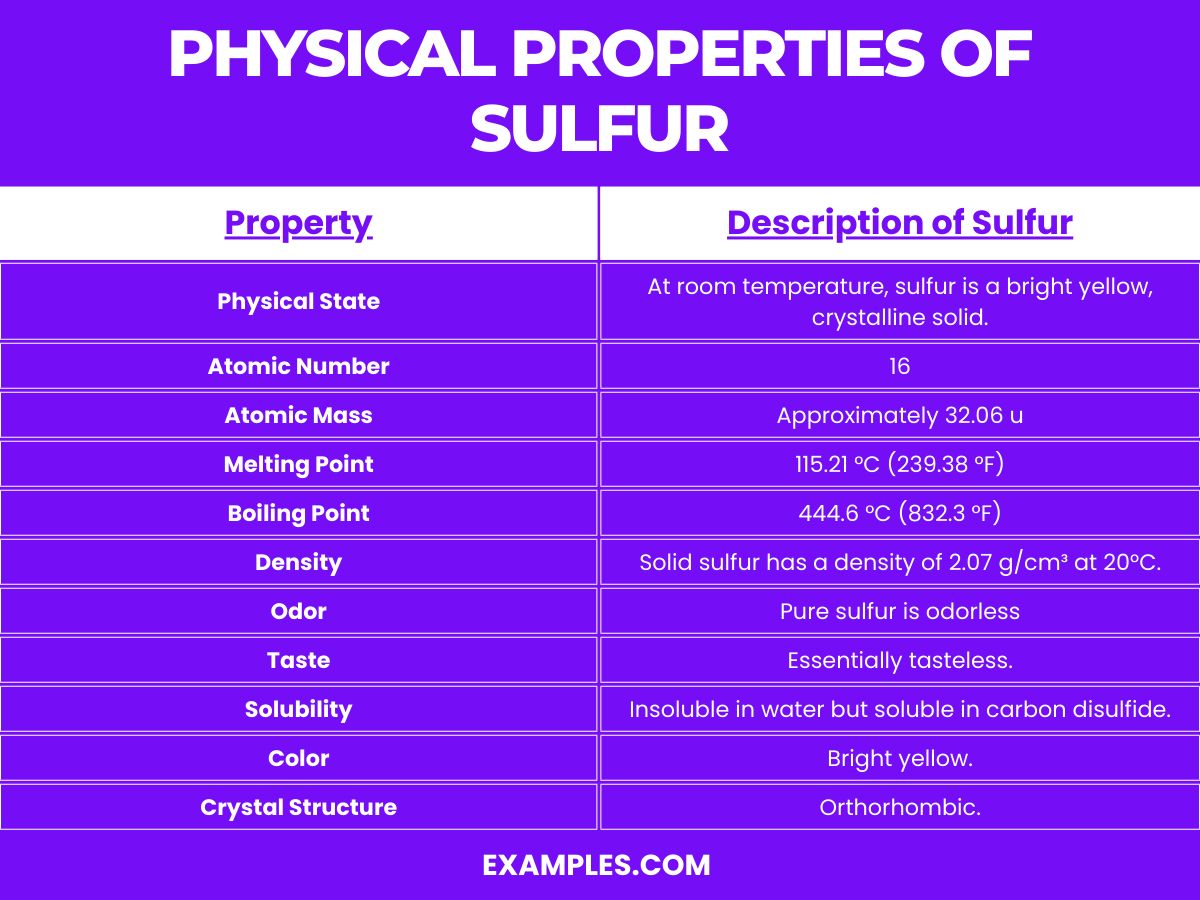
| Property | Description |
|---|---|
| Physical State | At room temperature, sulfur is a bright yellow, crystalline solid. |
| Atomic Number | 16 |
| Atomic Mass | Approximately 32.06 u |
| Melting Point | 115.21 °C (239.38 °F) |
| Boiling Point | 444.6 °C (832.3 °F) |
| Density | Solid sulfur has a density of 2.07 g/cm³ at 20°C. |
| Odor | Pure sulfur is odorless, but when combined with other elements, it may emit a distinct smell. |
| Taste | Essentially tasteless. |
| Solubility | Insoluble in water but soluble in carbon disulfide. |
| Color | Bright yellow. |
| Allotropes | Several allotropes exist, the most stable being octasulfur (S₈). |
| Crystal Structure | Orthorhombic. |
Sulfur, symbolized as ‘S’ and holding the atomic number 16, is a nonmetallic element known for its vibrant yellow coloration and prevalence in both the Earth’s crust and the cosmos. The chemical properties of sulfur are intricate and vital to many biological and industrial processes. Here, we provide a detailed exploration of sulfur’s chemical behavior:
1. Reactivity: Sulfur is known for its reactivity. It combines with almost all elements, except the noble gases, forming a variety of compounds known as sulfides. For instance, sulfur reacts with metals to form metal sulfides, which are often important ores. It also reacts with hydrogen to produce hydrogen sulfide (H₂S), a toxic and foul-smelling gas.
2. Oxidation States: Sulfur exhibits multiple oxidation states, including -2, +4, +6, and a few others, which are less common. The -2 state occurs when sulfur is found in sulfide minerals or hydrogen sulfide. The +4 and +6 states are seen in sulfur dioxide (SO₂) and sulfur trioxide (SO₃), respectively. These diverse oxidation states allow sulfur to form a wide range of compounds with varying properties.
3. Acidity: Sulfur compounds can exhibit acidity, particularly in sulfur oxides and oxyacids. Sulfur dioxide (SO₂) can dissolve in water to form sulfurous acid (H₂SO₃), and sulfur trioxide (SO₃) forms sulfuric acid (H₂SO₄) when combined with water. Sulfuric acid is a strong acid and a major industrial chemical used in various applications.
4. Combustion: When sulfur burns in oxygen, it forms sulfur dioxide (SO₂), a colorless gas with a sharp, choking smell. This is a common form of air pollution and is a significant byproduct of burning fossil fuels. The reaction is exothermic, releasing a considerable amount of energy.
5. Affinity to Hydrogen: Sulfur reacts with hydrogen to form hydrogen sulfide (H₂S), especially at high temperatures. Hydrogen sulfide is a colorless gas known for its characteristic ‘rotten egg’ smell. It is toxic and flammable, finding limited use in various industrial processes.
6. Allotropy: Sulfur exists in several allotropic forms. The most well-known allotrope is octasulfur (S₈), consisting of eight sulfur atoms arranged in a puckered ring. The allotropes of sulfur differ in their physical and chemical properties, influencing how sulfur reacts and forms compounds.
7. Role in Organic Compounds: Sulfur is a key element in many organic compounds, including amino acids, vitamins, and enzymes. Its ability to form stable C-S bonds is crucial in the biological function of these molecules. In addition, sulfur cross-links in proteins, such as those found in hair and feathers, are essential for their structural integrity.
These chemical properties highlight sulfur’s versatility and its importance across various domains, from industry to biology. Understanding these properties allows educators and students to appreciate the multifaceted roles that sulfur plays in both the natural world and human-made processes.
| Property | Description / Value |
|---|---|
| Melting Point | 115.21°C (rhombic), 119.0°C (monoclinic) |
| Boiling Point | 444.6°C |
| Thermal Conductivity | 0.205 W/(m·K) at 25°C (solid sulfur) |
| Specific Heat | 0.71 J/(g·K) at 25°C (solid sulfur) |
| Heat of Vaporization | 45 kJ/mol at boiling point |
| Heat of Fusion | 1.727 kJ/mol at melting point (rhombic) |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 2.07 g/cm³ (rhombic), 1.96 g/cm³ (monoclinic) |
| Mohs Hardness | 2 |
| Crystal Structure | Orthorhombic (rhombic), Monoclinic |
| Solubility | Insoluble in water, soluble in carbon disulfide |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Insulator, 10^-15 to 10^-17 S/m |
| Property | Description / Value |
|---|---|
| Atomic Number | 16 |
| Atomic Mass | 32.065 u |
| Neutron Cross Section | 0.53 barns (for ^32S) |
| Isotopes | ^32S (95.02%), ^33S (0.75%), ^34S (4.21%), ^36S (0.02%) |
| Radioactivity | Sulfur has no stable radioactive isotopes. ^35S is a radioactive isotope used in biological research, with a half-life of 87.32 days |
Sulfur forms a variety of compounds owing to its ability to exhibit different oxidation states and react with many elements. Here are some notable sulfur compounds:
Sulfur has four stable isotopes: ³²S, ³³S, ³⁴S, and ³⁶S. Here’s a detailed look at each:
The variation in isotopic composition of sulfur in different materials can be a powerful tool in tracing sources of sulfur or understanding processes in the Earth’s crust, atmosphere, and living systems. The study of sulfur isotopes has applications in paleoclimatology, oceanography, biology, and industrial source tracing. The isotopic composition in compounds like sulfates and sulfides can reveal information about the age, origin, or the history of the processes they have undergone.

Sulfur is a versatile element with a wide array of applications due to its chemical and physical properties. Here are some of the primary uses of sulfur:
The commercial production of sulfur is critical to many industries and is primarily sourced from the following methods:
Sulfur itself is a vital element for all life and is part of essential amino acids and proteins. However, certain compounds of sulfur can have varied effects on health:
In handling sulfur compounds, proper precautions and safety measures are essential to avoid adverse health effects.
Sulfur and its compounds play significant roles in various environmental processes, both beneficial and harmful:
Understanding the health and environmental impacts of sulfur and its compounds is critical in managing its applications and mitigating potential risks. This involves strict regulation of emissions, monitoring of occupational safety, and public awareness of its effects.
Sulfur is used in fertilizers, gunpowder, matches, insecticides, and pharmaceuticals. It’s vital for producing sulfuric acid, a key industrial chemical.
In its elemental form, sulfur is not toxic. However, compounds like hydrogen sulfide and sulfur dioxide can be hazardous at high concentrations.
Sulfur is crucial for synthesizing amino acids and proteins, but exposure to toxic sulfur compounds can cause respiratory and skin irritation.
Sulfur is abundant on Earth, commonly found near hot springs, volcanic regions, and in salt domes.
In food, sulfur is present in amino acids, vitamins like thiamine, and as additives like sulfites in dried fruits and wines.
Sulfur is typically found in volcanic and sedimentary deposits, near hot springs, and as a by-product of natural gas and petroleum processing.
Sulfur itself is not a salt, but it forms sulfide and sulfate salts, like sodium sulfate and iron sulfide, through chemical reactions.
Sulfur’s multifaceted nature is pivotal in both the environment and human health, necessitating careful handling and awareness. By understanding its properties, effects, and roles, we can harness its benefits while mitigating risks. This guide aims to empower educators and students with the knowledge to navigate sulfur’s complexities with informed caution and insight.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Sulfur?
14
16
18
20
Sulfur is most commonly found in which natural form?
Native element
Oxide compound
Sulfide mineral
Carbonate mineral
Which of the following is a major use of sulfur?
Insulation material
Semiconductor manufacturing
Fertilizer production
Optical lens manufacturing
Sulfur dioxide (SO2) is primarily used in:
Preserving fresh fruit
Making synthetic fibers
Water treatment
Vinegar production
Which property of sulfur is critical in vulcanizing rubber?
Elasticity
Conductivity
Reactivity with rubber
Color enhancement
At room temperature, sulfur is:
A liquid
A gas
A solid
Plasma
The most common oxidation state of sulfur in its compounds is:
-2
+2
+4
+6
Which environmental issue is associated with sulfur emissions?
Smog
Ozone depletion
Acid rain
Global warming
The "Claus process" is used in the industrial recovery of sulfur from:
Coal combustion
Coal combustion
Metal smelting
Petroleum refining
Sulfur is an essential element for:
Plants only
Animals only
Both plants and animals
Neither plants nor animals
Before you leave, take our quick quiz to enhance your learning!

