Hydrochloric acid is a solution of which gas in water?
Hydrogen
Chlorine
Hydrogen chloride
Hydrogen sulfide


Hydrochloric acid, often found in the fascinating world of chemistry, is a strong and important acid with a formula HCl. This clear, colorless solution is not just any acid; it plays a crucial role in our daily lives, from aiding in digestion within our stomachs to being used in various industries for cleaning and processing materials. Its unique properties make it a staple in science experiments and industrial applications alike, showcasing the power and versatility of chemistry in action. Whether it’s breaking down food or cleaning metal surfaces, hydrochloric acid demonstrates the incredible ways acids contribute to both nature and technology.
| Property | Value |
|---|---|
| Formula | HCl |
| Name | Hydrogen Chloride |
| Alternate Names | Anhydrous Hydrochloric Acid, Anhydrous Hydrogen Chloride, Chlorane |
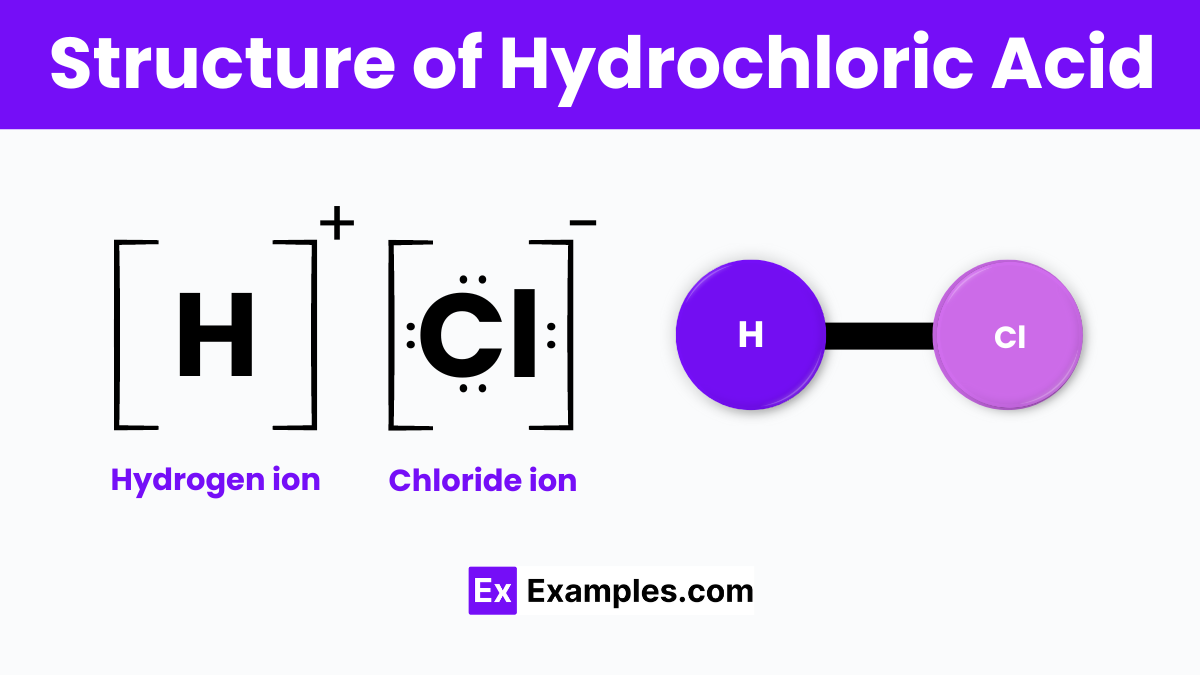
Hydrochloric Acid, commonly known as HCl, is a simple but powerful substance found in many scientific and industrial applications. Imagine it as a dance between two very different partners: a hydrogen atom (H) and a chlorine atom (Cl). These two atoms come together to form a molecule of HCl. In this molecular dance, the hydrogen atom donates its single electron to the chlorine atom, creating a strong bond that keeps them closely linked. This bonding results in HCl being a highly reactive, colorless gas under standard conditions, which, when dissolved in water, creates a strong and corrosive acid.
Hydrochloric Acid is like a recipe that combines two main ingredients: hydrogen gas (H₂) and chlorine gas (Cl₂). When these two gases are brought together, they react in an energetic dance to form Hydrochloric Acid. Picture it as if the hydrogen and chlorine atoms are eager to join hands, and when they do, they create HCl as a result. This reaction is not only fascinating but also quite straightforward. The chemical equation that represents this process looks like this:
In simpler terms, when one molecule of hydrogen gas reacts with one molecule of chlorine gas, two molecules of Hydrochloric Acid gas are formed. This reaction is typically carried out in a controlled environment, as it requires careful handling due to the reactive nature of chlorine gas and the corrosive property of Hydrochloric Acid. Once formed, Hydrochloric Acid can be dissolved in water to produce a solution that’s widely used in various applications, from cleaning to food processing.
| Property | Description |
|---|---|
| State at Room Temperature | Hydrochloric acid is typically found as a colorless to slightly yellow liquid when it is in a water solution. |
| Odor | It has a sharp, pungent smell, reminiscent of vinegar, but much stronger. |
| Taste | Not applicable and highly dangerous to try, but it is extremely sour. |
| Boiling Point | The boiling point of concentrated hydrochloric acid solution is around 110°C (230°F). |
| Melting Point | The melting point is about -27.32°C (-17.18°F) for the concentrated acid. |
| Solubility in Water | It is highly soluble in water, capable of forming a wide range of concentrations. |
| Density | The density of a 38% solution in water is approximately 1.19 g/cm³ at 25°C. |
| pH Value | A 1 M solution of HCl in water has a pH of about 0, making it a very strong acid. |
Hydrochloric Acid is an excellent conductor of electricity when dissolved in water. This is because it dissociates into ions (H⁺ and Cl⁻), which move freely in the solution, allowing it to conduct electricity.
HCl has a very low pH, making it a strong acid. A 1 Molar (1M) solution of HCl in water has a pH of around 0, indicating its high acidity.
While Hydrochloric Acid is stable under normal conditions, it can release dangerous chlorine gas when mixed with other chemicals, highlighting the importance of handling it with care.
| Property | Value |
|---|---|
| CAS Registry Number | 7647-01-0 |
| PubChem Compound ID | 313 |
| PubChem Substance ID | 24857783 |
| SMILES Identifier | Cl |
| InChI Identifier | InChI=1/ClH/h1H |
| RTECS Number | MW4025000 |
| MDL Number | MFCD00011324 |
| Property | Value |
|---|---|
| NFPA Health Rating | 3 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 1 |
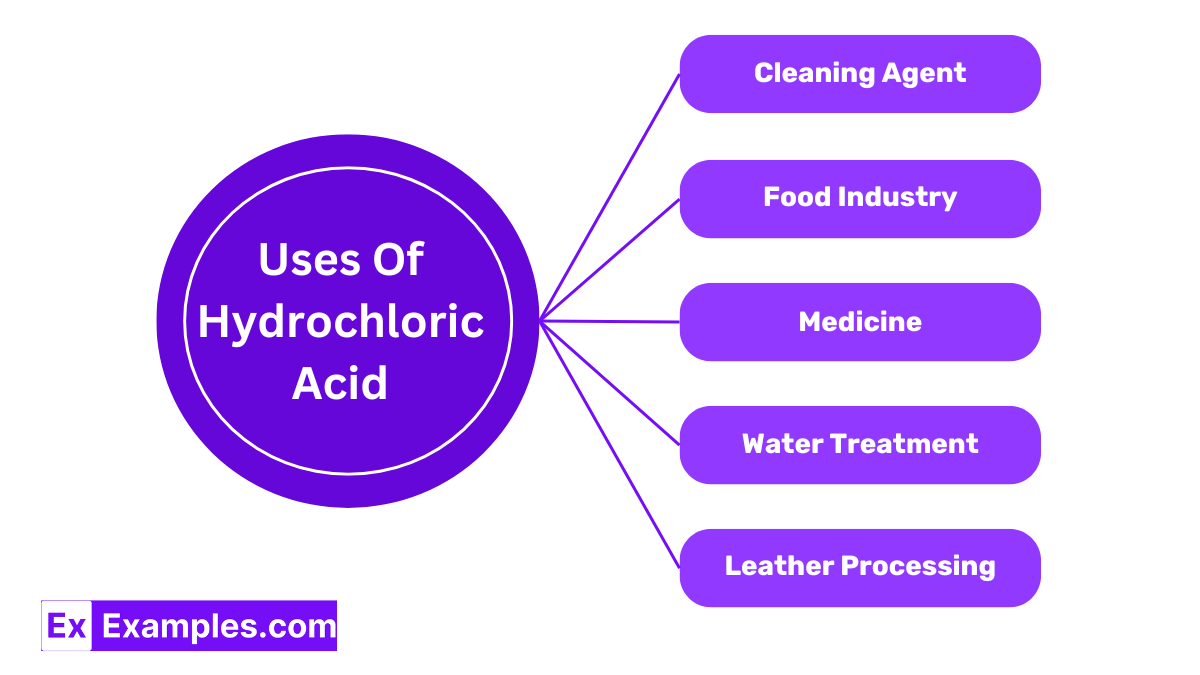
Hydrochloric Acid is widely used to clean metals before they are plated with another metal. Its strong acidic nature helps remove rust and other impurities from the surface of metals, ensuring that they are clean and ready for further processing.
In the food industry, HCl plays a crucial role in the production of various food products. It is used to make gelatin and other food additives. Hydrochloric Acid helps in breaking down proteins and is used in the process of making corn syrups.
Hydrochloric Acid is also an important component in the pharmaceutical industry. It is used to produce a variety of medicines and as a pH regulator in the formulation of some medications, ensuring they are effective when used.
In water treatment processes, Hydrochloric Acid is used to adjust the pH level of water. This is important in ensuring that drinking water is safe and in maintaining the effectiveness of the water purification process.
The leather industry uses Hydrochloric Acid in the tanning process of leather. It helps in removing impurities from the raw hide and preparing it for further processing, ensuring the leather is clean and ready for use.
In laboratories, HCl is a staple reagent. It is used in various chemical reactions and experiments due to its reactive nature. Hydrochloric Acid is also used in titrations to determine the concentration of basic (alkaline) solutions.
Diluted Hydrochloric Acid is found in many household cleaning products. It is effective in removing tough stains from toilets and tiles, making it a powerful cleaning agent for maintaining cleanliness in homes.
Yes, Hydrochloric Acid (HCl) can be harmful, causing skin burns, respiratory issues, and eye damage. Proper safety measures are essential when handling it.
Hydrochloric Acid is commonly found in industrial cleaners, the food industry for processing, and naturally in the stomach to aid digestion.
No, vinegar does not make Hydrochloric Acid. Vinegar mainly contains acetic acid, which is chemically different from Hydrochloric Acid (HCl).
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Hydrochloric acid is a solution of which gas in water?
Hydrogen
Chlorine
Hydrogen chloride
Hydrogen sulfide
Which of the following is a common use of hydrochloric acid?
Fertilizer production
Food preservation
Metal cleaning and pickling
Plastic manufacturing
What is the pH range of a concentrated hydrochloric acid solution?
1-2
3-4
5-6
7-8
Hydrochloric acid reacts with sodium hydroxide to produce which two compounds?
Sodium chloride and water
Sodium sulfate and water
Sodium nitrate and water
Sodium phosphate and water
What safety precaution is most important when handling hydrochloric acid?
Using fire-resistant gloves
Wearing chemical-resistant gloves and goggles
Wearing a dust mask
Using sunscreen
In the stomach, hydrochloric acid aids in digestion by activating which enzyme?
Amylase
Lipase
Pepsin
Trypsin
What is the molar mass of hydrogen chloride (HCl)?
36.46 g/mol
44.01 g/mol
58.44 g/mol
74.55 g/mol
Which industry heavily relies on hydrochloric acid for the production of polyvinyl chloride (PVC)?
Textile
Chemical
Food
Automotive
Hydrochloric acid is classified as which type of acid?
Weak acid
Strong acid
Organic acid
Lewis acid
What is the concentration of a standard concentrated hydrochloric acid solution?
6 M
10 M
12 M
15 M
Before you leave, take our quick quiz to enhance your learning!

