Berkelium was first synthesized in which year?
1945
1949
1952
1960
Dive into the enigmatic world of Berkelium, a synthetic element that embodies the frontier of nuclear chemistry and atomic science. Berkelium, with its profound significance in research and technology, illuminates the complexities of transuranic elements in the actinide series. This complete guide introduces you to the discovery, properties, and applications of Berkelium, showcasing its role in scientific breakthroughs and potential uses. Through engaging examples, we explore how Berkelium contributes to our understanding of the atomic world, highlighting its unique position in advancing materials science and nuclear research. Join us on a journey to uncover the secrets of Berkelium, an element that challenges the boundaries of chemistry and physics.
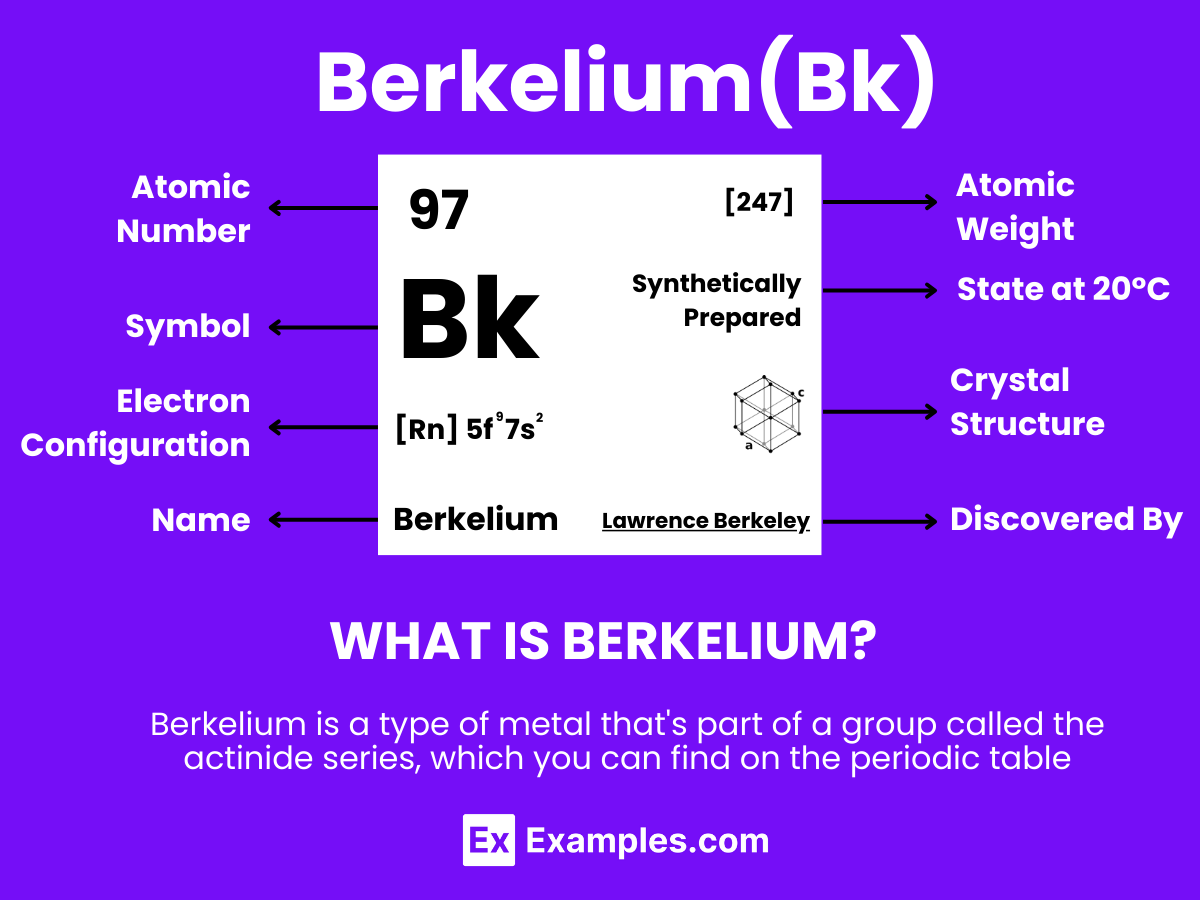
Berkelium is a synthetic, radioactive element with the symbol Bk and atomic number 97. belonging to the actinide series. It was discovered in 1949 by scientists at the University of California, Berkeley, which inspired its name. Produced through the bombardment of lighter elements in particle accelerators, berkelium does not occur naturally and has no stable isotopes, with Berkelium-247 being its most stable isotope, featuring a half-life of approximately 1,380 years. Primarily used in scientific research, particularly in the synthesis of heavier transuranic and transactinide elements, its most notable application was in the creation of Tennessine. Berkelium’s chemistry, characterized by its ability to exhibit +3 and +4 oxidation states, is similar to that of other actinides, yet its practical applications are limited due to its radioactivity and rarity.
| Actinium | Fermium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
Berkelium, a synthetic element with the symbol Bk and atomic number 97, belongs to the actinide series on the periodic table. Understanding its atomic structure provides insights into its chemical behavior and applications in science and technology.
| Property | Value |
|---|---|
| Appearance | Silvery, lustrous metal |
| Phase at Room Temperature | Solid |
| Density | 14.78 g/cm³ (alpha form at room temperature) |
| Melting Point | 986°C |
| Boiling Point | Approximately 2,600°C (estimated) |
| Atomic Mass | 247 u (most stable isotope) |
| Crystal Structure | Hexagonal close-packed (hcp) |
| Hardness | Relatively soft (comparable to other actinides) |
Berkelium (Bk) is a fascinating element in the actinide series, with the atomic number 97. It showcases unique chemical properties due to its position in the periodic table, nestled among the heavy, radioactive elements. Berkelium’s chemical behavior is characterized by its electron configuration and its ability to exhibit various oxidation states, most notably +3 and +4. Let’s delve into the detailed chemical properties of Berkelium, highlighting its reactions and compounds.
| Property | Value |
|---|---|
| Melting Point | 986°C |
| Boiling Point | Approximately 2,600°C (estimated) |
| Heat of Fusion | 7.92 kJ/mol (estimated) |
| Heat of Vaporization | 290 kJ/mol (estimated) |
| Specific Heat Capacity | Unknown, similar to other actinides |
| Property | Value |
|---|---|
| State | Solid (at room temperature) |
| Density | 14.78 g/cm³ |
| Appearance | Silvery, metallic, tarnishes in air |
| Crystal Structure | Face-centered cubic (estimated) |
| Property | Value |
|---|---|
| Electrical Resistivity | High, specific value unknown |
| Magnetic Ordering | Paramagnetic at room temperature |
| Thermal Conductivity | Low, specific value unknown |
| Property | Value |
|---|---|
| Most Stable Isotope | Berkelium-247 (half-life: 1,380 years) |
| Primary Decay Modes | Alpha decay, spontaneous fission |
| Neutron Cross Section | High, specific values vary by isotope |
| Critical Mass | Not well-defined, due to high radioactivity and scarcity |
The preparation of Berkelium (Bk), a synthetic and radioactive element of the actinide series, involves complex nuclear reactions and requires a high-energy neutron source. Here is an overview of the standard process used to prepare Berkelium:
Description: A binary oxide where Berkelium is in the +4 oxidation state, showcasing its ability to form stable oxides.
Equation: 2Bk+O₂→BkO₂
Description: A halide compound with Berkelium in the +3 oxidation state, used in the study of Berkelium’s chemical properties.
Equation: 3Bk+23Cl₂→BkCl₃
Description: A fluoride where Berkelium is in +3 oxidation state, crucial for investigating its ionic radii in solid state.
Equation: 3Bk+23F₂→BkF₃
Description: A compound forming dark crystals, indicating Berkelium’s reactivity with halogens in the +3 oxidation state.
Equation: 3Bk+23I₂→BkI₃
Description: An oxide where Berkelium is primarily in the +3 oxidation state, essential for understanding its basic chemistry.
Equation: 2Bk+23O₂→Bk2O₃
Description: A sulfate compound demonstrating Berkelium’s ability to form complex anionic compounds in the +3 oxidation state.
Equation: 2Bk+3H₂SO₄→Bk₂(SO₄)₃+3H₂
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Berkelium-245 | 4.94 days | Alpha decay |
| Berkelium-246 | 1.8 days | Alpha decay, spontaneous fission |
| Berkelium-247 | 1,380 years | Alpha decay |
| Berkelium-248 | >9 years | Alpha decay, spontaneous fission |
| Berkelium-249 | 330 days | Beta decay to Californium-249 |
| Berkelium-250 | 3.212 hours | Beta decay, spontaneous fission |
Berkelium, a synthetic and radioactive element, has very specialized uses primarily in scientific research due to its scarcity and radioactivity. Here are some of its applications:
The production of Berkelium (Bk), a synthetic and highly radioactive element, is a complex process that primarily takes place in nuclear reactors. Berkelium-249, the most accessible isotope of Berkelium, is produced through several neutron capture reactions involving heavier elements like Plutonium (Pu), Americium (Am), or Curium (Cm). Here’s a step-by-step breakdown of the typical production process:
Despite its challenges, Berkelium has several notable applications, primarily in scientific research:
This article has meticulously outlined the intricate properties of Berkelium, spanning its physical, chemical, thermodynamic, material, electromagnetic, and nuclear aspects. Through comprehensive tables, we’ve delved into Berkelium’s characteristics, underscoring its significance in scientific research and its unique position within the actinide series, despite its challenges in handling and rarity.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Berkelium was first synthesized in which year?
1945
1949
1952
1960
What is the symbol for berkelium on the periodic table?
Be
Bk
Br
Ba
Berkelium belongs to which group in the periodic table?
Transition metals
Lanthanides
Actinides
Halogens
What is the atomic number of berkelium?
93
94
96
97
Which method was used to synthesize berkelium for the first time?
Nuclear fusion
Particle accelerator bombardm
Chemical reaction
Electrolysis
What is the primary use of berkelium?
Fuel for nuclear reactors
Catalyst in chemical reactions
Research purposes
Production of alloys
Which university is associated with the discovery of berkelium?
Harvard University
Stanford University
University of California, Berkeley
MIT
Berkelium has how many protons?
95
96
97
98
The isotope berkelium-249 has a half-life of approximately:
1 day
10 days
330 days
5 years
Berkelium is classified as what type of element?
Metal
Metalloid
Non-metal
Noble gas
Before you leave, take our quick quiz to enhance your learning!

