What is the Van der Waals equation used for?
Describing ideal gases
Describing real gases
Calculating heat capacity
Determining reaction rates

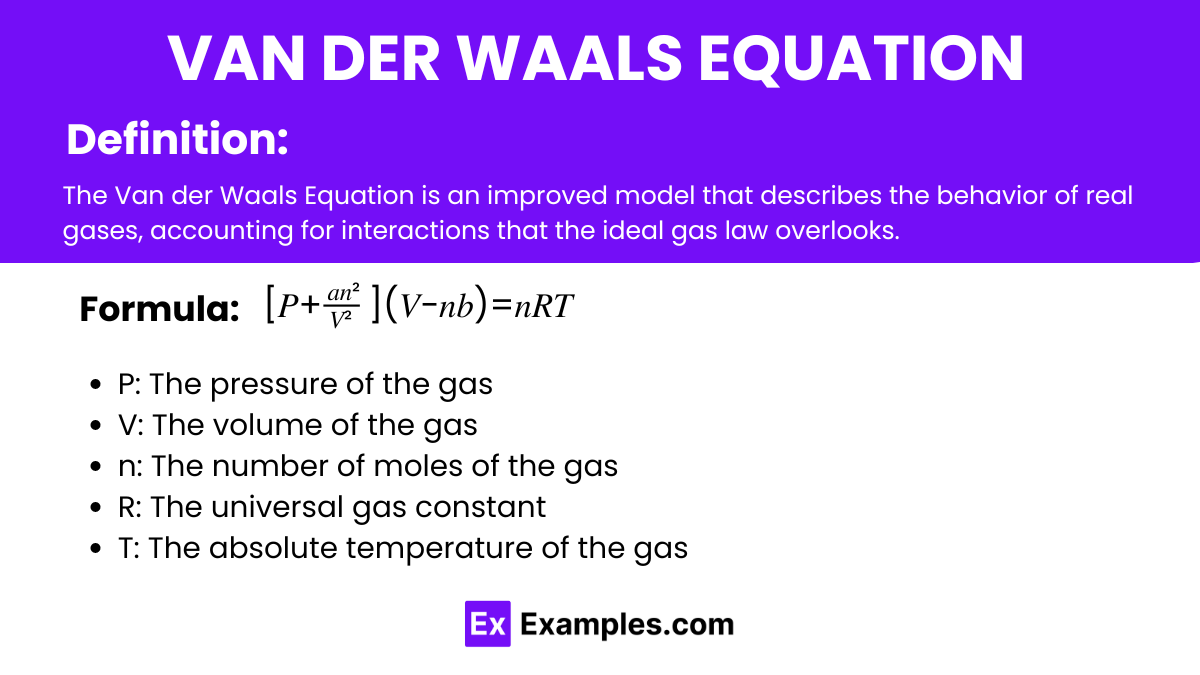
The Van der Waals Equation is an improved model that describes the behavior of real gases, accounting for interactions that the ideal gas law overlooks. It introduces corrections for the finite size of gas molecules and the intermolecular forces between them.
The formula for the Van der Waals Equation is:
Here’s a breakdown of what each term represents:
The units for the Van der Waals constants are:
The Van der Waals Equation modifies the ideal gas law to account for the non-ideal behavior of real gases by considering the volume of gas molecules and the attractive forces between them. Here’s how to derive the Van der Waals Equation:
Ideal Gas Law: Begin with the ideal gas law: 𝑃𝑉=𝑛𝑅𝑇 where:
Correction for Finite Volume (b): Real gas molecules have a finite size, meaning they occupy some of the container’s volume, reducing the volume available for movement. The volume correction subtracts 𝑛𝑏 from the total volume: 𝑉′=𝑉−𝑛𝑏 where:
Correction for Attractive Forces (a): Real gas molecules attract each other, reducing the impact of collisions with the container walls. This results in a lower pressure than predicted by the ideal gas law. Van der Waals introduced a correction by adding a pressure term: 𝑃′=𝑃+𝑎𝑛²/𝑉² where:
This pressure correction reflects the reduction in pressure due to intermolecular attractions.
Combine the Corrections: Substitute the corrected pressure and volume back into the ideal gas law:
(𝑃+𝑎𝑛²/𝑉²)(𝑉−𝑛𝑏)=𝑛𝑅𝑇
This is the Van der Waals Equation, which provides a more accurate description of gas behavior, particularly at high pressures and low temperatures, compared to the ideal gas law.
The Van der Waals equation of state provides a more accurate representation of real gas behavior compared to the ideal gas law. It incorporates corrections for the finite size of gas molecules and the attractive forces between them.
In the Van der Waals equation, the volume correction addresses the finite size of gas molecules. In reality, gas molecules occupy a certain amount of space, reducing the effective volume available for gas movement compared to an ideal gas where the molecules are considered point particles with no volume.
Key Aspects of the Volume Correction:
Concept:
The volume occupied by gas molecules themselves cannot be used for free movement. This means that the effective volume available for gas movement is less than the total volume of the container.
Correction Term:
The volume correction is represented by the parameter 𝑏, which accounts for the volume occupied by one mole of gas molecules. The effective volume available for movement is then reduced by subtracting the term 𝑛𝑏, where 𝑛 is the number of moles of gas.
Implication in the Equation:
The Van der Waals equation replaces the volume term of the ideal gas law with a corrected term, reducing the available volume by the amount occupied by gas molecules.
Thus, the volume correction allows the equation to account for the finite size of gas molecules and provides a more accurate representation of real gas behavior than the ideal gas law.
In the Van der Waals equation, the pressure correction accounts for the attractive forces between gas molecules, which are neglected in the ideal gas law. These attractive forces cause gas molecules to experience less effective pressure than would be predicted by the ideal gas law.
Key Aspects of the Pressure Correction:
Concept:
In real gases, molecules attract one another. This attraction reduces the impact that molecules have on the container walls because they tend to cluster together, resulting in a lower effective pressure.
Correction Term:
The pressure correction is represented by the parameter 𝑎, which quantifies the strength of the intermolecular attractive forces. The correction increases the measured pressure to account for this reduced impact due to attraction between molecules.
Implication in the Equation:
The Van der Waals equation includes an additional term to adjust the pressure upward by adding a factor that depends on 𝑎 and the number of moles of gas. This pressure correction helps to more accurately predict the actual pressure of real gases compared to the ideal gas law.
Overall, the pressure correction ensures that the Van der Waals equation reflects the reality of intermolecular attractions and provides a better model of real gas behavior.
The Ideal Gas Equation and the Van der Waals Equation are both equations of state that describe the behavior of gases, though they do so with different assumptions.
Ideal Gas Equation:
The Ideal Gas Equation assumes that gas molecules do not have volume and do not exert attractive or repulsive forces on each other. The equation is expressed as:
𝑃𝑉=𝑛𝑅𝑇
where:
This equation works well under conditions where gases behave ideally, typically at high temperatures and low pressures.
Van der Waals Equation:
The Van der Waals Equation modifies the Ideal Gas Equation to account for the non-ideal behavior of real gases. It introduces two corrections:
The Van der Waals Equation is expressed as:
(𝑃+𝑎𝑛²/𝑉²)(𝑉−𝑛𝑏)=𝑛𝑅𝑇
where:
The Van der Waals Equation provides a more accurate model for gas behavior. Especially under high pressure and low temperature conditions where gases deviate significantly from ideal behavior.
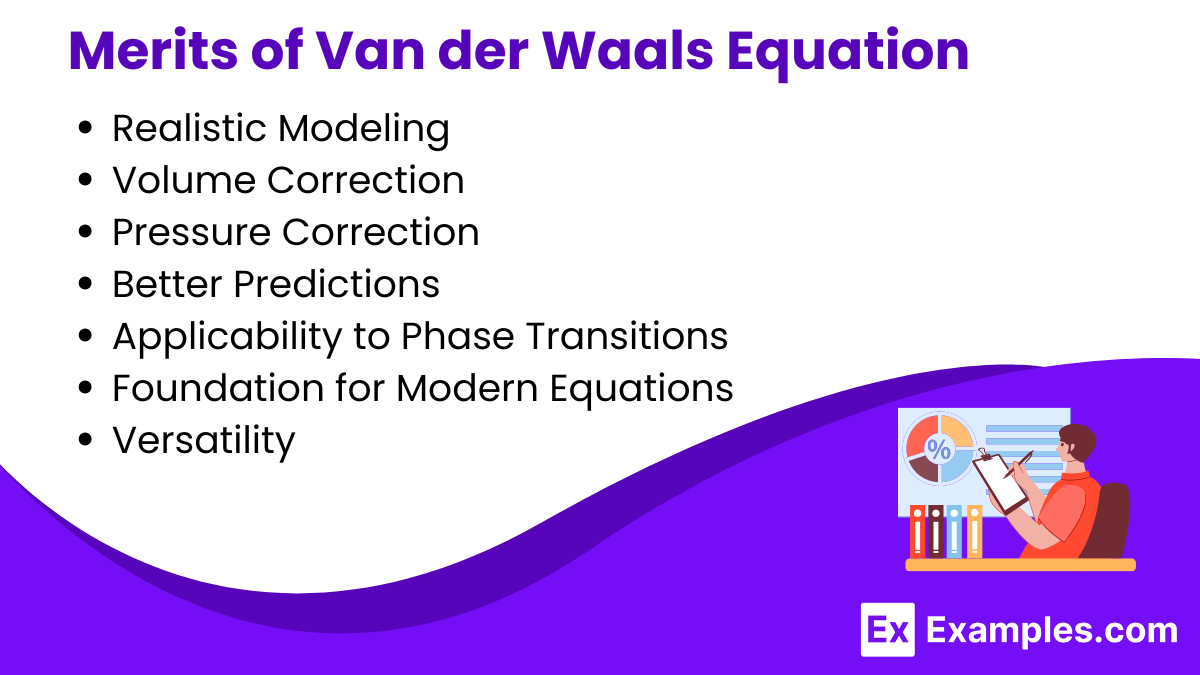
The Van der Waals equation of state is a more realistic model for describing the behavior of gases compared to the ideal gas law. Here are some of its key merits:
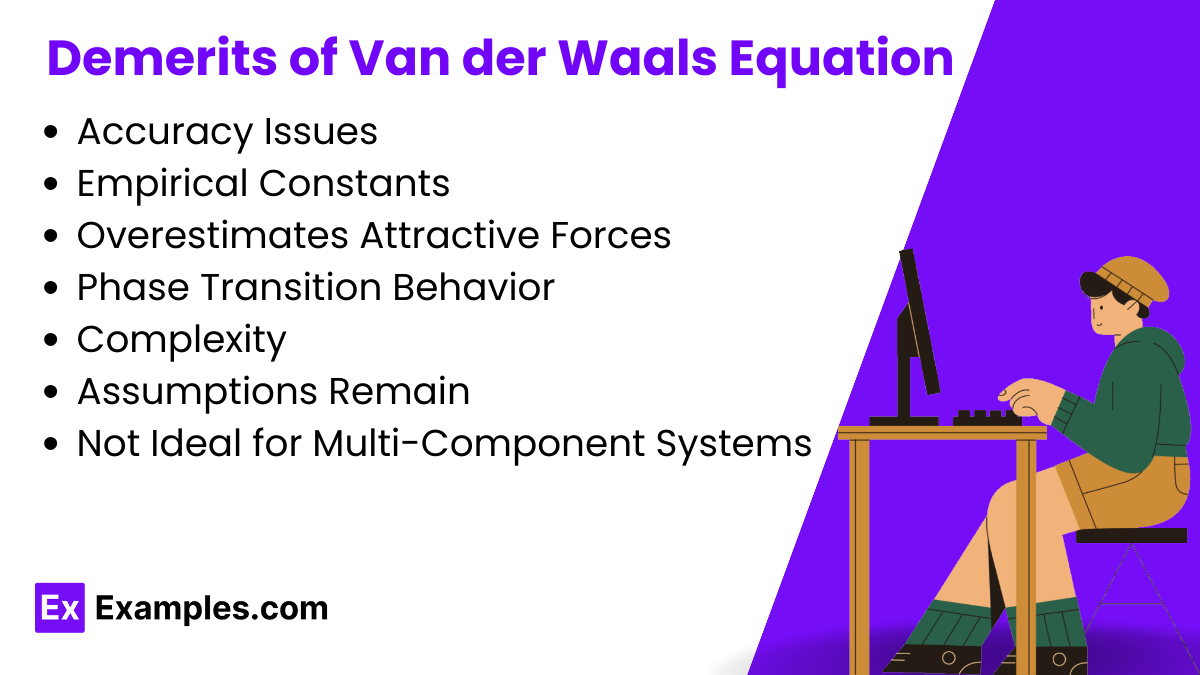
While the Van der Waals equation provides a more accurate representation of real gas behavior than the ideal gas law, it still has some limitations:
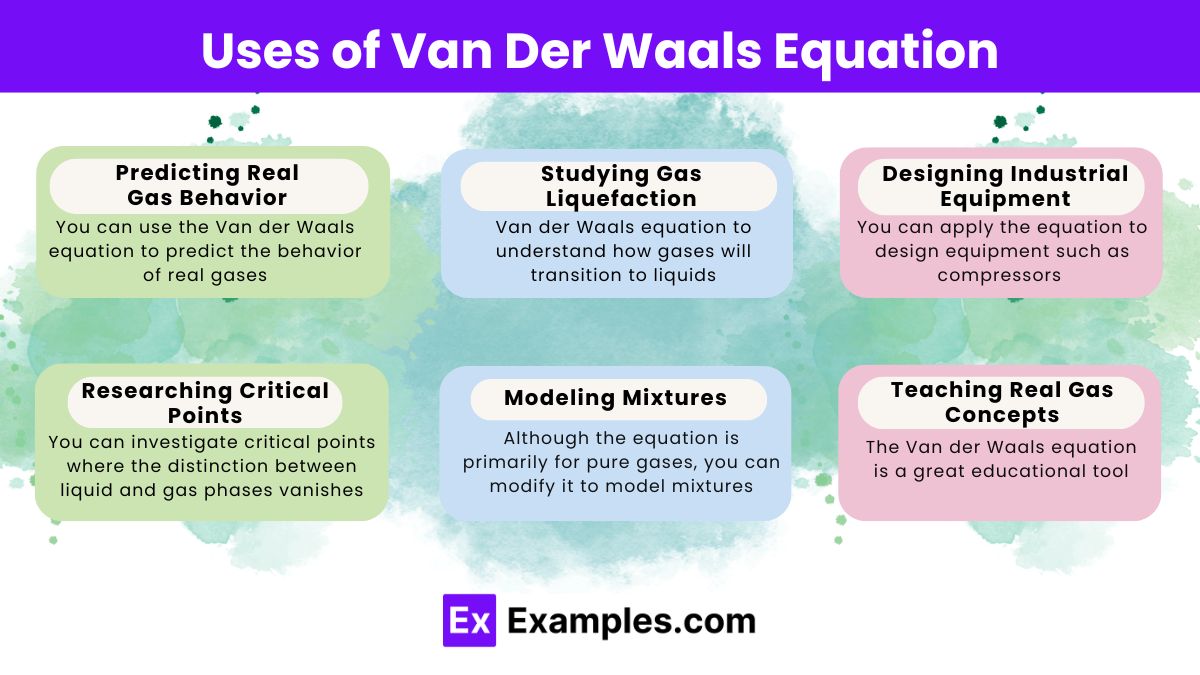
The Van der Waals equation improves upon the ideal gas law by accounting for real gas behavior. Here are some ways you can use this equation:
The Van der Waals Equation of State adjusts the ideal gas law to account for gas molecule size and intermolecular forces.
It incorporates corrections for gas particle volume and attractive forces, providing a more accurate representation of real gas behavior.
It’s applied to describe the behavior of real gases, especially at high pressures or low temperatures, where deviations from ideality are significant.
The equation includes parameters 𝑎 and 𝑏, representing intermolecular attraction and molecular volume, respectively, for a specific gas.
By considering the volume occupied by gas particles and intermolecular attractions, it accurately predicts deviations from ideal gas behavior at high pressures.
Yes, it accounts for changes in gas volume and pressure during phase transitions. Such as condensation or liquefaction.
While it’s more accurate than the ideal gas law. It’s less complex than some equations, striking a balance between simplicity and accuracy.
It aids in understanding real gas behavior. Contributing to the design of processes in chemical engineering, refrigeration, and other fields.
Experimental data, such as critical temperature and pressure, are used to determine the Van der Waals constants for specific gases.
Yes, by considering deviations from ideal behavior. The Van der Waals Equation can be adapted to describe non-ideal solutions and mixtures.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the Van der Waals equation used for?
Describing ideal gases
Describing real gases
Calculating heat capacity
Determining reaction rates
In the Van der Waals equation, what does the constant 'a' account for?
Volume of gas molecules
Intermolecular attractions
Temperature dependence
Molar mass
In the Van der Waals equation, what does the constant 'b' account for?
Pressure correction
Intermolecular repulsion
Finite volume of gas molecules
Temperature correction
How does the Van der Waals equation modify the ideal gas law?
It introduces a temperature correction factor
It adds terms for intermolecular forces and molecular volume
It changes the units of pressure and volume
It includes a universal gas constant
What happens to the Van der Waals equation at very high temperatures and low pressures?
It reduces to the ideal gas law
It becomes invalid
It shows significant deviations from the ideal gas law
It becomes linear
What does the term 'P + a/V²' in the Van der Waals equation represent?
Effective pressure
Ideal pressure
Corrected volume
Effective temperature
What does the term 'V - b' in the Van der Waals equation represent?
Effective pressure
Ideal volume
Corrected volume
Effective temperature
Which of the following is a limitation of the Van der Waals equation?
It does not account for temperature changes
It cannot be used for liquids
It is only accurate at very high pressures
It does not account for molecular interactions
How are the constants 'a' and 'b' in the Van der Waals equation determined?
They are universal constants
They are determined experimentally for each gas
They depend on temperature and pressure
They are derived from the ideal gas law
What effect does a high value of 'a' have on a gas according to the Van der Waals equation?
The gas behaves more ideally
The gas has strong intermolecular attractions
The gas molecules occupy more volume
The gas is less compressible
Before you leave, take our quick quiz to enhance your learning!

