What is the atomic number of Nihonium?
113
114
115
116
DDive into the captivating world of Nihonium (Nh), the 113th element in the periodic table, with our comprehensive guide. Nihonium, a synthetic wonder, opens up new avenues in scientific research and technology. This introduction offers you a detailed exploration of its discovery, unique properties, potential uses, and groundbreaking compounds. Enriched with practical examples, our guide seamlessly blends scientific rigor with accessibility, making it perfect for both enthusiasts and professionals eager to uncover the mysteries of Nihonium.
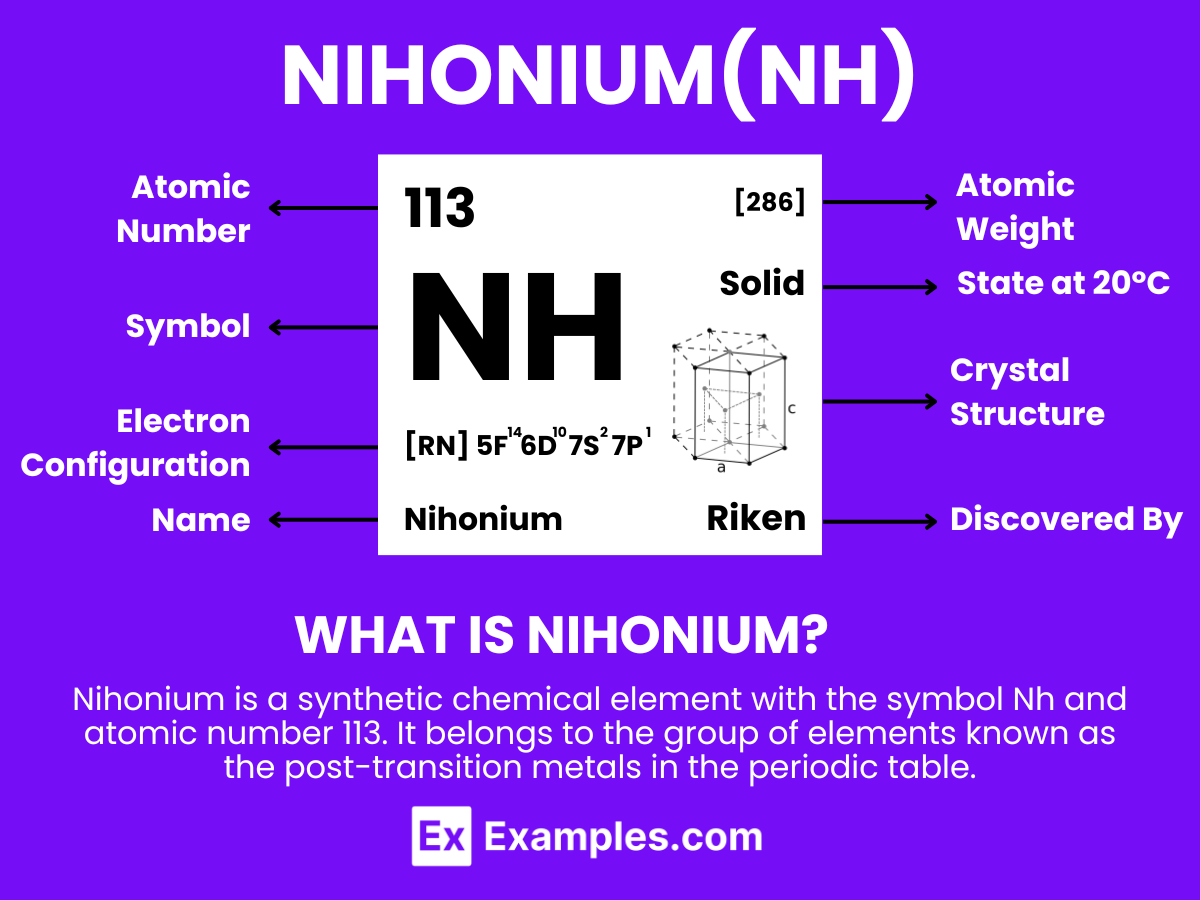
Nihonium is a synthetic chemical element with the symbol Nh and atomic number 113. It belongs to the group of elements known as the post-transition metals in the periodic table. Nihonium was first discovered in 2004 by a team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, and later confirmed by a team of Japanese scientists at the RIKEN institute. It is named after Japan (Nihon in Japanese), marking it as the first element on the periodic table to be named after an Asian country.
| Meitnerium | Darmstadtium | Roentgenium |
| Copernicium | Flerovium | Moscovium |
| Livermorium | Tennessine | Oganesson |
Nihonium is not encountered in its gaseous state naturally and is a synthetic element that has only been produced in laboratory settings. Like molybdenum, the atomic structure of Nihonium as an element—including its electrons, protons, and neutrons—applies across all its hypothetical physical states (solid, liquid, gas).
Nihonium (Nh) has an atomic number of 113, meaning it possesses 113 protons in its nucleus. The number of neutrons in its most stable isotope, Nihonium-286, is 173, giving it a mass number of 286 (113 protons + 173 neutrons). The electrons are arranged in orbitals around the nucleus. The theoretical electron configuration of Nihonium is [Rn] 5f¹⁴ 6d¹⁰ 7s² 7p¹, indicating it has two electrons in the 7s orbital, one electron in the 7p orbital, and a completely filled 5f and 6d orbitals, beyond the filled orbitals of Radon (Rn), a noble gas.
113 protons in the nucleus, giving it its unique elemental properties.
173 neutrons in its most stable isotope, contributing to the mass of the atom.
Electrons arranged in orbitals, with the theoretical electron configuration of [Rn] 5f¹⁴6d¹⁰7s²7p¹, reflecting its position in the periodic table as a superheavy, post-transition metal.
| Property | Value |
|---|---|
| Atomic Number | 113 |
| Atomic Weight | Most stable isotope: 286 |
| Melting Point | Estimated to be around 700 K (430 °C, 806 °F) |
| Boiling Point | Estimated to be around 1430 K (1157 °C, 2115 °F) |
| Density | Predicted to be unknown |
| Phase at Room Temperature | Solid (predicted) |
| Crystal Structure | Predicted structure unknown |
| Color | Unknown, likely synthetic appearance |
Nihonium (Nh) is a synthetic element with atomic number 113, making it one of the superheavy elements. Its chemical properties are not extensively known due to its extremely short half-life and the difficulty in producing sufficient quantities for experimental analysis. However, theoretical predictions based on its position in the periodic table (group 13, period 7, p-block) provide some insights into its potential chemical behavior.
Nihonium (Nh) is a synthetic element that does not occur naturally and must be created in a laboratory environment. The process of synthesizing Nihonium involves highly sophisticated equipment and techniques, primarily through the collision of lighter atomic nuclei. Here’s a simplified overview of the method used to prepare Nihonium:
1.Nihonium Dioxide (NhO₂)
2.Nihonium Tetrafluoride (NhF₄)
3.Nihonium Heptoxide (Nh₂O₇)
4.Nihonium Sulfide (Nh₂S)
5.Nihonium Hexacarbonyl (Nh(CO)₆)
6.Nihonium Chloride (NhCl₃)
| Isotope | Atomic Number | Number of Neutrons | Half-life |
|---|---|---|---|
| Nh-284 | 284 | 172 | 0.9 milliseconds |
| Nh-285 | 285 | 173 | 4.8 milliseconds |
| Nh-286 | 286 | 174 | 9.5 milliseconds |
| Nh-287 | 287 | 175 | 23 milliseconds |
| Nh-288 | 288 | 176 | 37 milliseconds |
Nihonium (Nh) is a synthetic element with atomic number 113 on the periodic table. It’s part of the group known as the superheavy elements, specifically located in the post-transition metals category. Discovered in 2004 by a team of Russian and American scientists, nihonium does not occur naturally and is created in a laboratory through the fusion of lighter elements. Due to its extremely short half-life and the difficulty in producing it, nihonium’s uses are primarily limited to scientific research. Here are some potential and theoretical uses of nihonium:
In the far future, the properties of nihonium and other superheavy elements could be investigated for their potential use in space exploration technologies, such as propulsion systems or radiation shielding. The element’s nuclear characteristics might offer novel solutions to current challenges in long-duration space missions.
Nihonium (Nh) is a synthetic element with the atomic number 113. It does not occur naturally in the environment and is produced artificially in a laboratory. The production of Nihonium is a complex process, involving sophisticated equipment and highly controlled conditions. The element was first recognized and reported by a joint team of Russian and American scientists working at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, and later independently by a team of Japanese scientists at the Riken Institute.
The primary method for producing Nihonium involves nuclear fusion reactions. These reactions typically involve bombarding a target material made of a lighter element with ions of a heavier element. The choice of target and projectile ions is crucial, as it determines the likelihood of the fusion reaction leading to the production of Nihonium.
In the case of Nihonium, one of the most successful methods has been the bombardment of Americium (Am) targets with Zinc (Zn) ions. The reaction can be represented as follows:
This reaction involves the collision of Americium-243 nuclei with Zinc-48 ions, potentially leading to the formation of Nihonium-291 after the emission of a neutron. The success rate of such experiments is extremely low due to the small cross-section for fusion, meaning that many attempts are necessary to produce even a few atoms of Nihonium.
Hot fusion processes are typically employed, where the projectile ion is accelerated to high energies before impacting the target. This approach increases the kinetic energy involved in the collision, facilitating the overcoming of the Coulomb barrier – the repulsive force between the positively charged nuclei.
Unlike hot fusion, cold fusion involves reactions with lower projectile energies, which lead to the production of compound nuclei at lower excitation energies. However, cold fusion has not been as successful for the production of superheavy elements like Nihonium due to lower probabilities of fusion and survival of the produced compound nucleus.
Nihonium (Nh), with atomic number 113, is a synthetic element in the periodic table that was first recognized by the International Union of Pure and Applied Chemistry (IUPAC) in 2016. Its properties are not well-studied due to its extremely short half-life and the difficulty in producing it, which limits practical applications. However, its discovery has implications in various scientific fields, and hypothetical applications have been proposed based on the properties of other elements in its group (the post-transition metals) and its position in the periodic table. Here are some potential applications of Nihonium:
Nihonium represents a monumental achievement in the realm of superheavy element research, offering theoretical insights despite practical challenges related to its synthesis and stability. Its discovery enriches our understanding of the periodic table and nuclear physics, potentially paving the way for future scientific breakthroughs in various fields, from material science to targeted medical therapies.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Nihonium?
113
114
115
116
What is the chemical symbol for Nihonium?
Nh
Ni
Nn
Nm
Nihonium belongs to which group in the periodic table?
Group 12
Group 13
Group 14
Group 15
Nihonium is classified as which type of element?
Alkali metal
Transition metal
Post-transition metal
Metalloid
Which of the following is a property of Nihonium?
It is highly reactive with water
It is a noble gas
It is radioactive
It is non-metallic
In which year was Nihonium officially recognized as a new element?
2005
2009
2012
2016
Nihonium was first discovered by researchers from which country?
United States
Germany
Japan
Russia
What is the most stable isotope of Nihonium?
Nh-284
Nh-285
Nh-286
Nh-287
What type of decay does Nihonium primarily undergo?
Alpha decay
Beta decay
Gamma decay
Neutron emission
Which of the following is a predicted property of Nihonium based on its position in the periodic table?
High electrical conductivity
High melting point
Metallic character
Non-reactivity with acids
Before you leave, take our quick quiz to enhance your learning!

