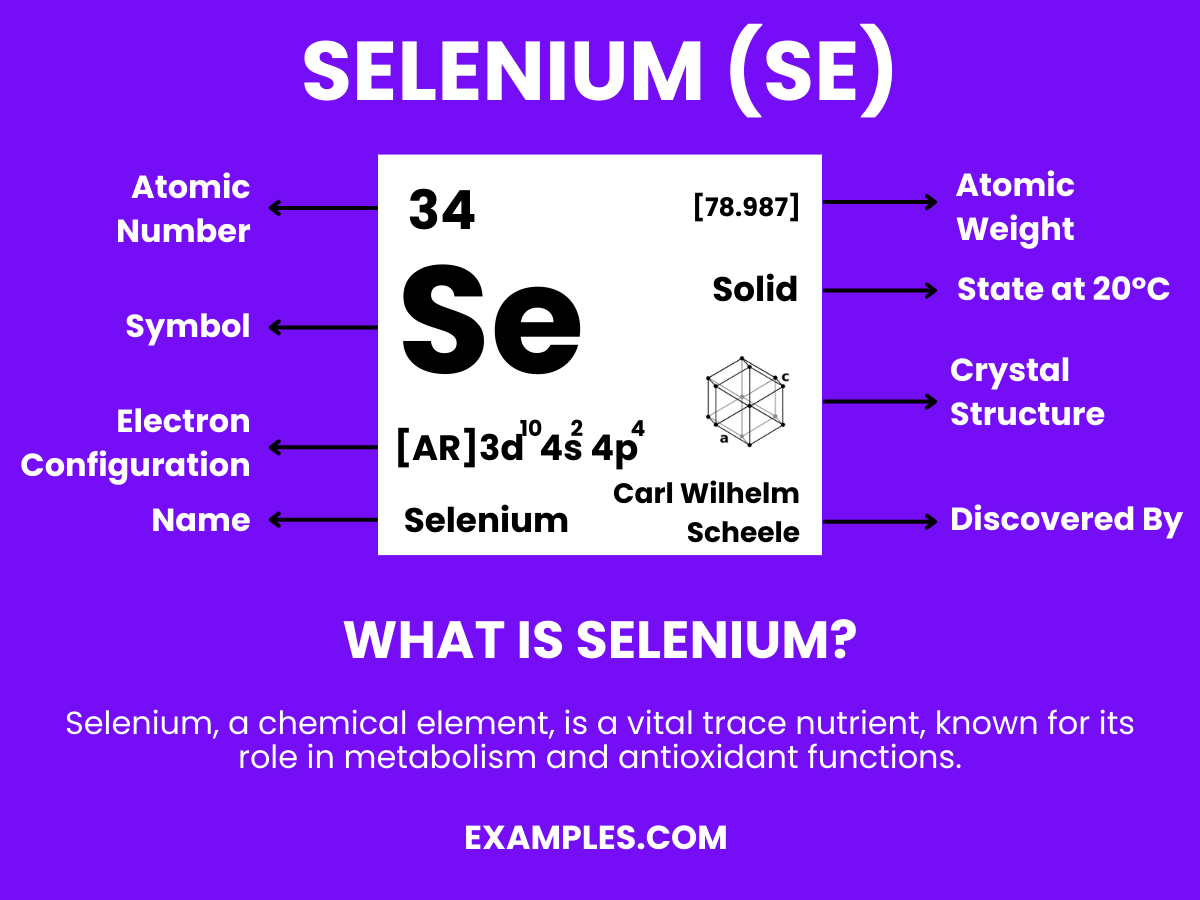
What is the atomic number of Selenium?
32
34
36
38
Selenium, often associated with technology, is as vital as Hydrogen is in the universe of elements. This complete guide is a treasure trove of examples that illuminate the diverse applications and functionalities of Selenium. From automating web browsers to testing across various platforms, this resource is packed with insights and techniques. Ideal for educators aiming to bring cutting-edge technology into their curriculum, the guide ensures a comprehensive understanding, fostering an environment of innovation and expertise in the classroom.
Selenium is a powerful suite of tools that supports rapid development of test automation for web-based applications. Originally developed for testing web browsers, Selenium has evolved into a robust framework used by developers and testers to automate browser tasks and verify web application functionality. Its versatility makes it a favorite among tech educators and students alike. With its open-source nature, Selenium allows for customization and scaling, making it an adaptable tool for various educational and professional projects. Simple yet powerful, Selenium is an indispensable tool in the digital age.
| Hydrogen | Phosphorus |
| Carbon | Sulfur |
| Nitrogen | Chlorine |
| Oxygen | Bromine |
| Fluorine | Iodine |
Formula: Se₈
Composition: Eight selenium atoms.
Bond Type: Weak single bonds forming a ring structure.
Molecular Structure: Ring or chain-like depending on allotrope.
Electron Configuration: Six valence electrons per atom, forty-eight in total for Se₈.
Significance: Essential micronutrient for animals and humans, plays a role in preventing cell damage and boosting immunity.
Role in Chemistry: Used in electronics, glassmaking, and as a nutritional supplement. Found in various allotropic forms, with red and gray being the most common.
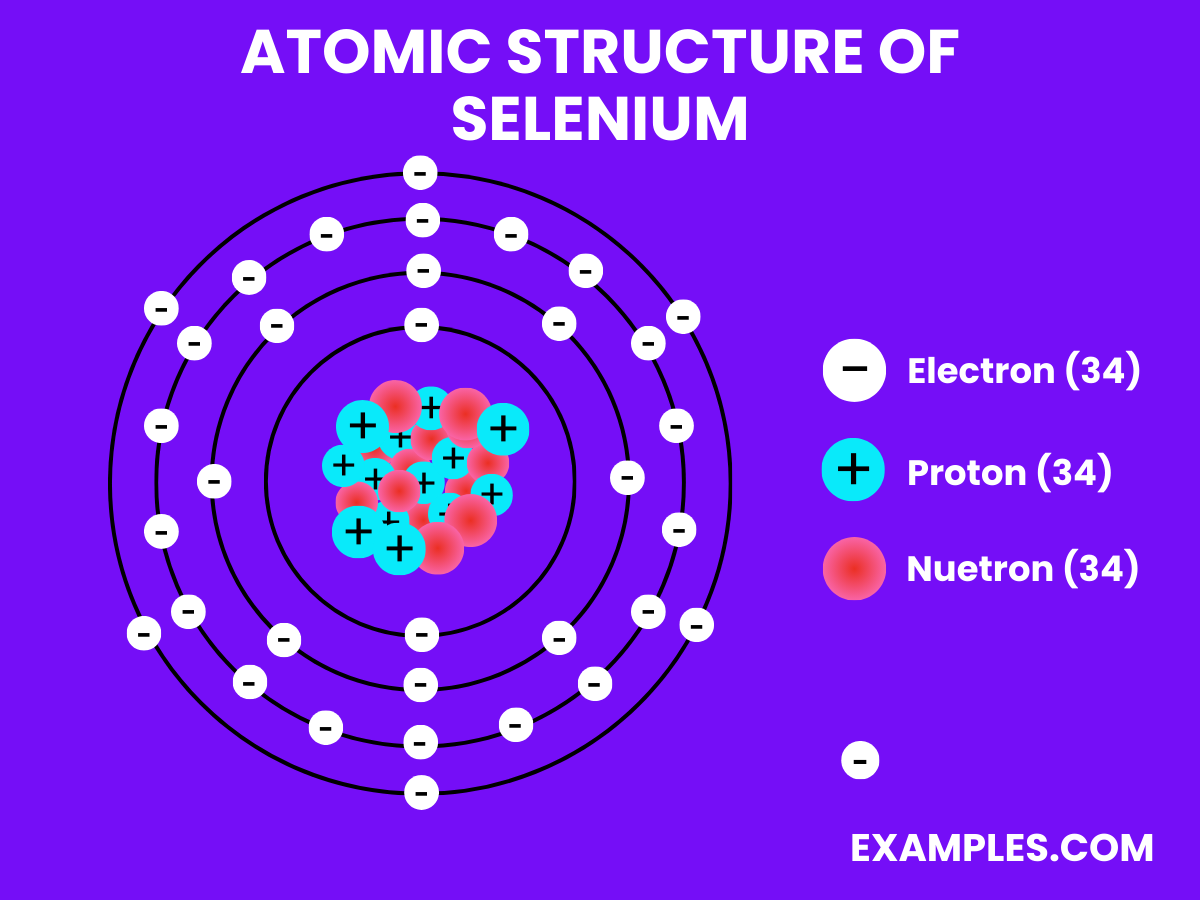
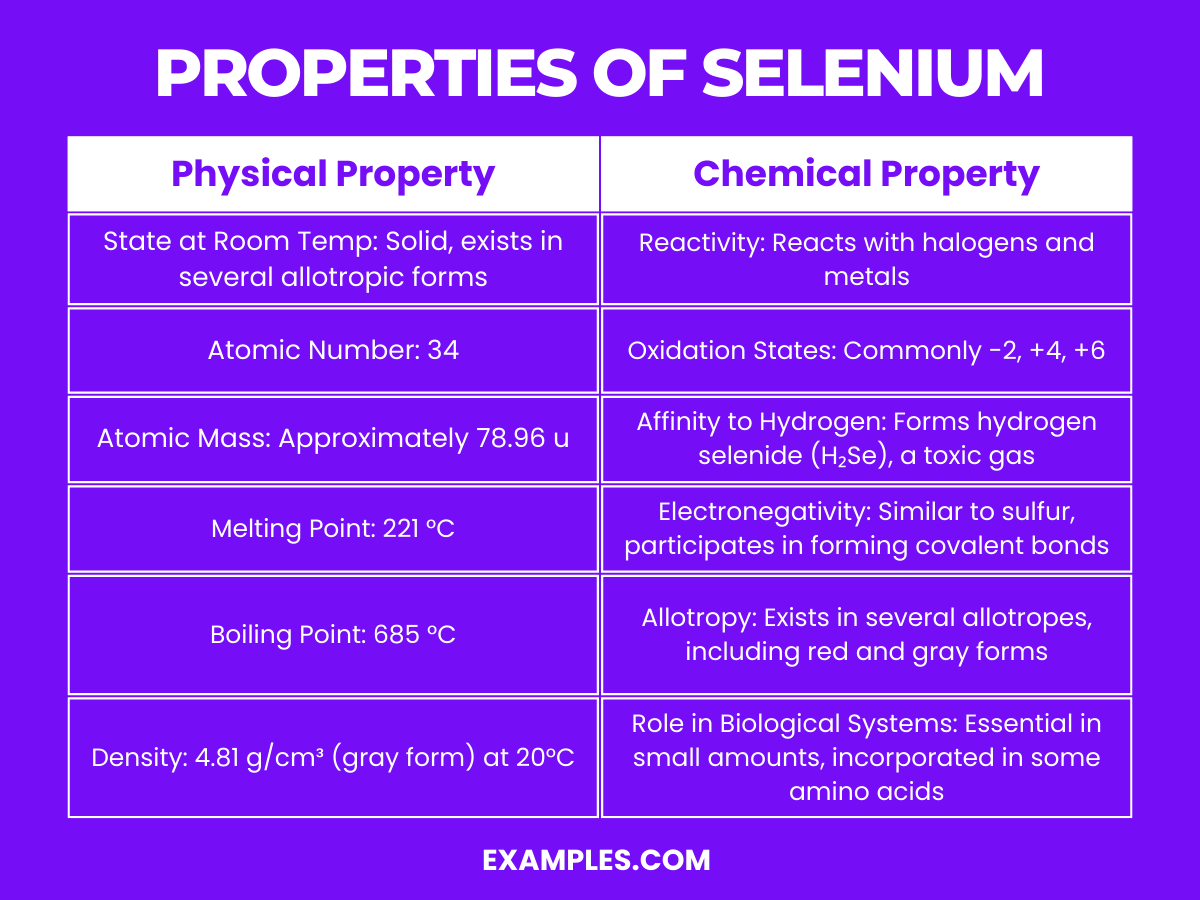
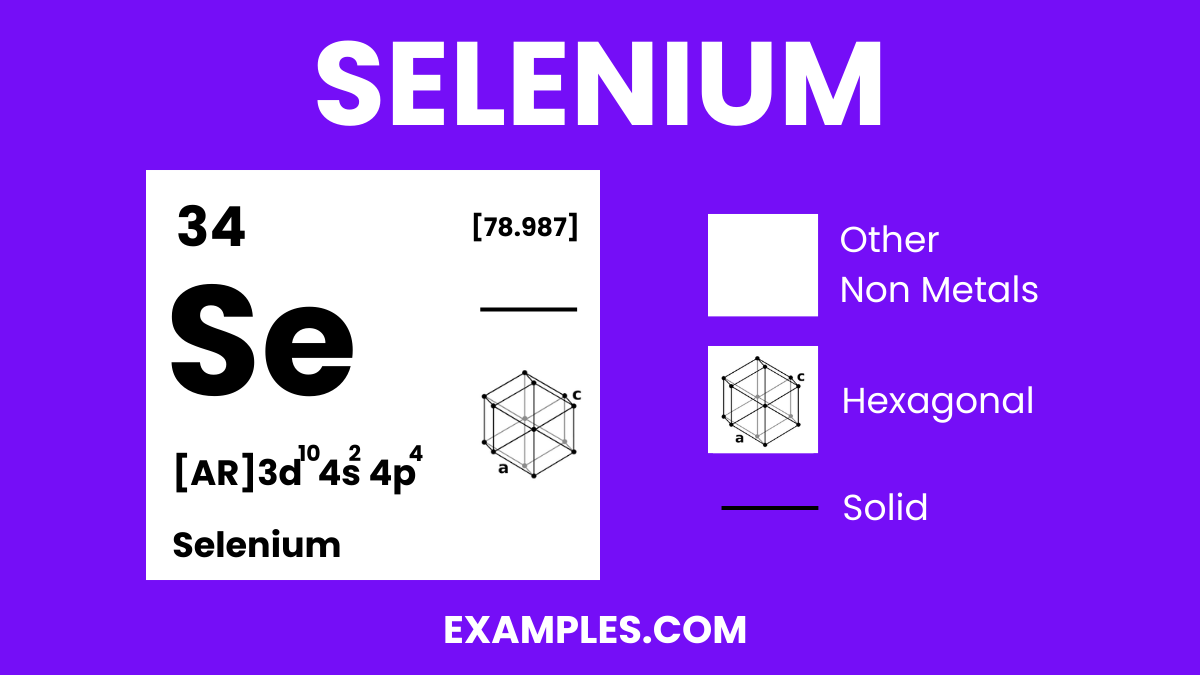
Selenium is a non-metal with intriguing properties, and understanding its physical characteristics is crucial for various applications. Here are the physical properties presented in a table for clarity:
| Physical Property | Description |
|---|---|
| State at Room Temperature | Exists in several allotropic forms, the most stable being a gray metallic-looking solid |
| Atomic Number | 34 |
| Atomic Mass | Approximately 78.971 u |
| Melting Point | 221 °C (430 °F) |
| Boiling Point | 685 °C (1,265 °F) |
| Density | Gray selenium: 4.81 g/cm³ |
| Color | Varies with allotropes; gray, red, and black are common |
| Conductivity | Displays photoconductivity; electrical conductivity increases with light exposure |
| Solubility | Insoluble in water, but soluble in carbon disulfide |
Selenium’s chemical behavior is complex and varied due to its ability to exist in multiple oxidation states and its similarity to sulfur. Here are detailed explanations of its chemical properties:
1. Reactivity and Compounds: Selenium forms compounds with many elements and exhibits multiple oxidation states, including -2, +4, and +6. In the -2 state, it forms selenides with metals similar to how sulfur forms sulfides. In the +4 and +6 states, it forms compounds like selenium dioxide (SeO₂) and selenium trioxide (SeO₃).
2. Allotropy: Selenium exists in several allotropes, the most stable being gray selenium, which has a metallic look and is a semiconductor. The red and black allotropes are less stable and have different physical properties.
3. Acidity and Basicity: Selenium can exhibit both acidic and basic behavior depending on its state and what it is reacting with. For instance, hydrogen selenide (H₂Se) is a weak acid.
4. Combustion: Selenium burns in the air to form selenium dioxide (SeO₂), a colorless, irritating to the respiratory system gas.
5. Photoconductivity: One of the most notable chemical properties of selenium is its photoconductivity, meaning its electrical conductivity increases when light is shone on it. This property is utilized in photocopier drums and solar cells.
Understanding these properties of selenium, both physical and chemical, is crucial for its application in electronics, glassmaking, and nutritional supplements. Its unique behavior, especially in response to light, makes it an important element in various technological and biological processes.
| Property | Description / Value |
|---|---|
| Melting Point | 221°C (430°F) for gray selenium |
| Boiling Point | 684°C (1263°F) |
| Thermal Conductivity | 0.52 W/(m·K) at 25°C (gray selenium) |
| Specific Heat | 0.321 J/(g·K) at 25°C (gray selenium) |
| Heat of Vaporization | 95.48 kJ/mol at boiling point |
| Heat of Fusion | 6.69 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 4.81 g/cm³ (gray selenium) |
| Young’s Modulus | 10 GPa (gray selenium) |
| Tensile Strength | 50 MPa (brittle, gray selenium) |
| Mohs Hardness | 2 (gray selenium) |
| Elastic Modulus | ~10 GPa (gray selenium) |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 1 x 10^-5 S/m (gray selenium) |
| Photoconductivity | Selenium increases in electrical conductivity when light is shone on it, making it a semiconductor |
| Property | Description / Value |
|---|---|
| Atomic Number | 34 |
| Atomic Mass | 78.971 u |
| Neutron Cross Section | 11.7 barns (for ^78Se) |
| Isotopes | Stable isotopes include ^74Se, ^76Se, ^77Se, ^78Se, ^80Se, ^82Se |
| Radioactivity | Selenium has several radioactive isotopes, such as ^75Se with a half-life of 119.78 days |
Selenium, a nonmetal element with the symbol Se and atomic number 34, is known for its diverse chemical forms and compounds. Here are some key compounds of selenium:
| Isotope | Symbol | Atomic Mass | Natural Abundance | Half-Life | Notes |
|---|---|---|---|---|---|
| Selenium-74 | ⁷⁴Se | 73.9224764 | 0.89% | Stable | Non-radioactive, used in studies due to its stable nature |
| Selenium-76 | ⁷⁶Se | 75.9192136 | 9.37% | Stable | Commonly used in industrial applications |
| Selenium-77 | ⁷⁷Se | 76.9199140 | 7.63% | Stable | Utilized in NMR spectroscopy due to its nuclear spin |
| Selenium-78 | ⁷⁸Se | 77.9173091 | 23.77% | Stable | Predominant isotope, used in various research areas |
| Selenium-79 | ⁷⁹Se | 78.9184991 | – | 3.27 × 10⁵ years | Radioactive, produced in nuclear fission |
| Selenium-80 | ⁸⁰Se | 79.9165213 | 49.61% | Stable | Heaviest stable isotope, widely used in medical and biological research |
| Selenium-82 | ⁸²Se | 81.9166994 | 8.73% | 1.08 × 10²⁰ years | Very long half-life, subject of double beta decay studies |
Selenium is a trace mineral that plays a vital role in many bodily functions and has a wide array of uses both in human health and various industries:
Selenium is found in a variety of foods, and including these in your diet can help maintain optimal levels of this essential mineral:
Understanding the uses, dietary sources, and commercial applications of selenium helps in appreciating its role in health and industry. Incorporating selenium-rich foods into the diet and recognizing its wider applications shows its multifaceted importance.
Selenium is a trace mineral essential to human health, but it is required only in small amounts. Its impact on health is substantial, and understanding its effects is crucial for maintaining a balanced diet and preventing health issues.
Selenium, while vital in trace amounts, can have significant environmental impacts, particularly when its concentration in a given ecosystem exceeds natural levels due to human activities.
Understanding the dual nature of selenium’s effects is crucial for health professionals, environmentalists, and policy-makers. While it’s a necessary nutrient for health, careful consideration and management are needed to prevent its negative implications on health and the environment.
When selenium reacts, it forms compounds, often with a red or gray color, and can produce a pungent, decaying odor.
Selenium is used in electronics, glass production, and as a dietary supplement for its antioxidant properties.
Selenium is found in metal selenides, selenates, selenides in soils, and organic compounds like selenoproteins.
Chemical forms of selenium include elemental selenium, selenide, selenate, and selenite.
Selenium is a substance, specifically a nonmetal chemical element, identifiable by the symbol Se and atomic number 34.
Selenium is a vital trace element with a powerful duality: essential for health in small quantities yet potentially harmful in excess. Balancing its intake is crucial for human well-being and environmental health. Understanding, monitoring, and managing selenium levels are vital, requiring informed choices in diet, industry practices, and environmental policies to harness its benefits and mitigate risks.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Selenium?
32
34
36
38
Selenium is an essential trace element primarily known for its role in:
Enhancing muscle function
Improving skin health
Boosting the immune system
Strengthening bones
Which form of selenium is commonly used in dietary supplements?
Sodium selenate
Selenium dioxide
Selenium sulfide
Selenomethionine
Selenium is most effectively absorbed in the human body as:
Elemental selenium
Selenocysteine
Selenide ions
Selenium oxides
What is the role of selenium in electronic applications?
Semiconductor manufacturing
Insulator in capacitors
Conductor in cables
Battery acid regulation
Which environmental concern is associated with selenium?
Air pollution
Water contamination
Soil degradation
Noise pollution
Which is NOT a physical property of selenium?
Metallic luster
High malleability
Brittle texture
Grey coloration
The discovery of selenium was made by:
Dmitri Mendeleev
Jöns Jacob Berzelius
Marie Curie
Antoine Lavoisier
Selenium toxicity is primarily due to:
Overexposure to sunlight
Ingestion of high doses
Inhalation of fumes
Skin contact
Which industry heavily relies on selenium for manufacturing?
Textile
Automotive
Glass
Food processing
Before you leave, take our quick quiz to enhance your learning!

