What is the atomic number of Iodine?
51
53
55
57
Discover the intriguing world of iodine, an essential element in both nature and the classroom. This guide dives deep into its properties, uses, and significance, providing you with well-rounded content to enrich your lessons. As you teach about the role of iodine in human health and the environment, remember it’s as fundamental as hydrogen in many chemical reactions. Engage your students with captivating examples and clear explanations, making chemistry relatable and exciting.
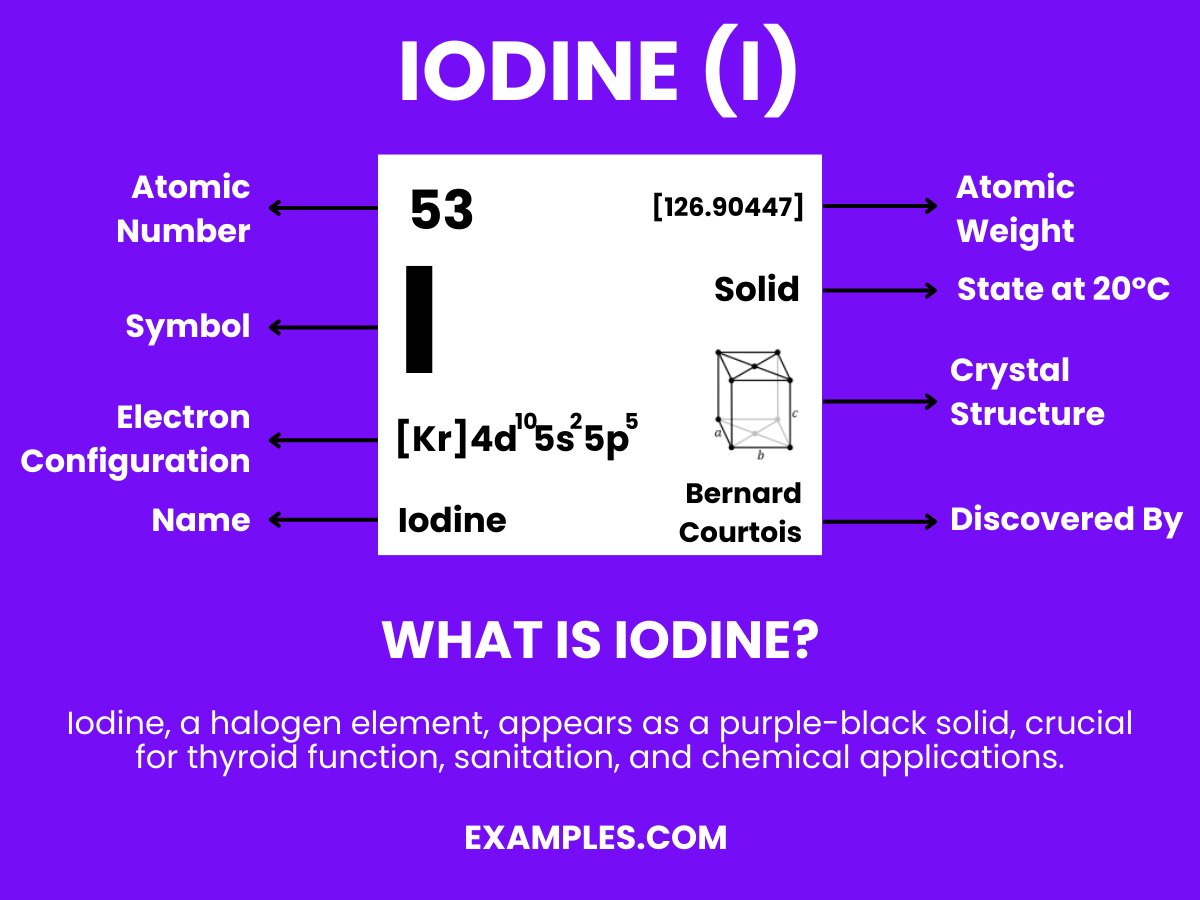
Iodine is a chemical element with the symbol “I” and atomic number 53. It’s a non-metallic, dark-gray or purple-black, lustrous solid at room temperature. Iodine is essential in biology, notably in the synthesis of thyroid hormones in the human body, which regulate metabolism and other important functions. It’s also used widely in medicine, nutrition, and various industrial applications. Teachers can explain iodine’s significance by highlighting its critical role in maintaining human health and its diverse uses in our daily lives.
| Hydrogen | Phosphorus |
| Carbon | Sulfur |
| Nitrogen | Chlorine |
| Oxygen | Selenium |
| Fluorine | Bromine |
Formula: I₂
Composition: Two iodine atoms.
Bond Type: A single covalent bond connecting the atoms.
Molecular Structure: Diatomic molecule.
Electron Configuration: Seven valence electrons per atom, fourteen in total for I₂.
Significance: Essential nutrient for thyroid function and widely used as a disinfectant and antiseptic.
Role in Chemistry: Used in the synthesis of certain organic compounds and in iodometry. Appears as a solid crystal at room temperature with a distinctive purple vapor.
| Physical Property | Description |
|---|---|
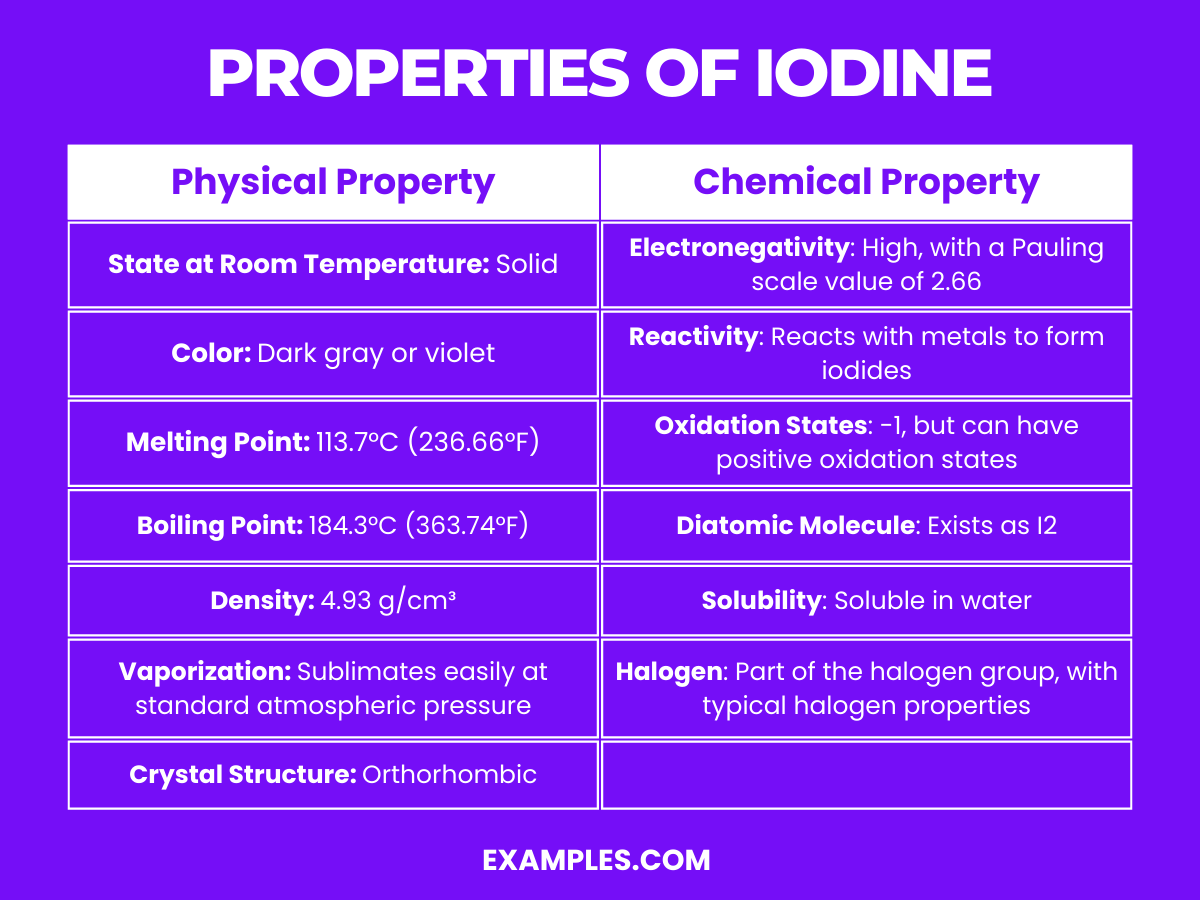
| State at Room Temperature | Solid, usually appears as shiny, dark gray or violet crystals. |
| Color | Dark gray or violet, depending on the form and purity. |
| Melting Point | 113.7°C (236.66°F), where solid iodine begins to transition into a liquid form. |
| Boiling Point | 184.3°C (363.74°F), the temperature at which it converts from a liquid to a gaseous state. |
| Density | 4.93 g/cm³ at 20°C, relatively high density for a non-metallic element. |
| Vaporization | Sublimates easily, meaning it transitions directly from a solid to a gaseous state at standard atmospheric pressure. |
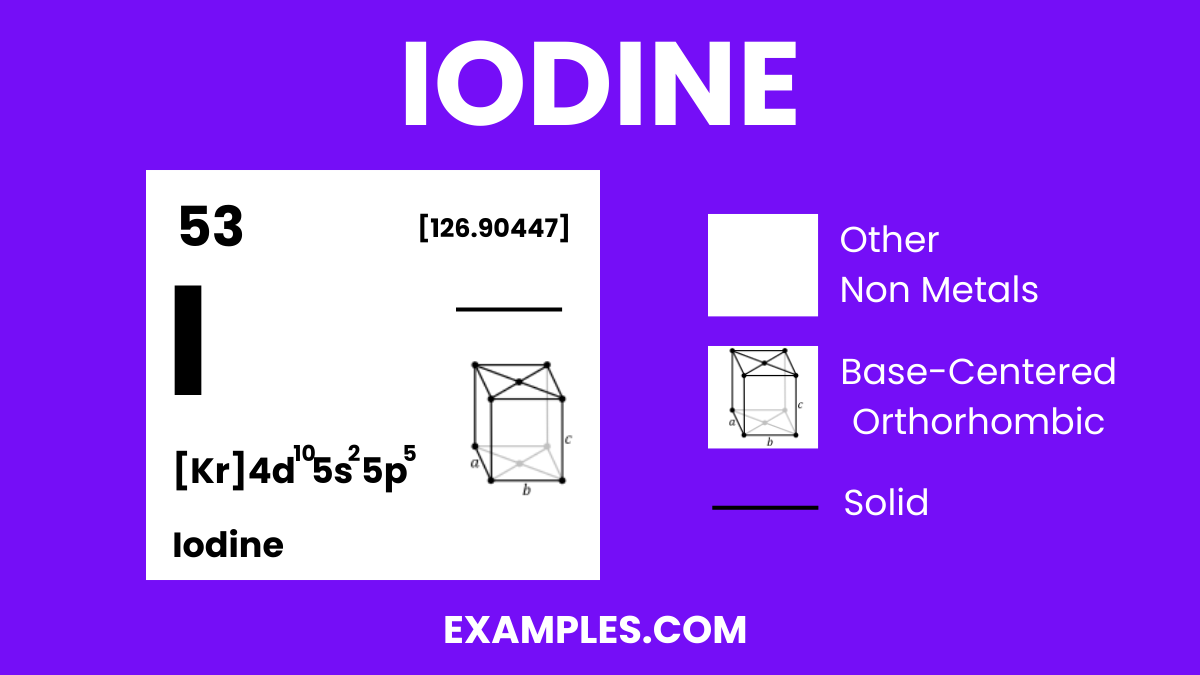
| Crystal Structure | Orthorhombic, forming lattice structures in its solid form. |
| Appearance | Iodine crystals are shiny and lustrous with a metallic sheen, signifying its crystalline solid state. |
By understanding both the physical and chemical properties of iodine, scientists and manufacturers can use it effectively in various applications, from medical treatments and dietary supplements to chemical synthesis and industrial processes.
| Property | Description / Value |
|---|---|
| Melting Point | 113.7°C (236.66°F) |
| Boiling Point | 184.3°C (363.74°F) |
| Thermal Conductivity | 0.449 W/(m·K) at 25°C |
| Specific Heat | 0.214 J/(g·K) at 25°C |
| Heat of Vaporization | 41.57 kJ/mol at boiling point |
| Heat of Fusion | 15.52 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 4.933 g/cm³ at 20°C |
| Color | Shiny, metallic-gray solid or violet vapor |
| Solubility in Water | 0.03 g/100 mL at 20°C |
| Crystal Structure | Orthorhombic |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Poor conductor of electricity |
| Property | Description / Value |
|---|---|
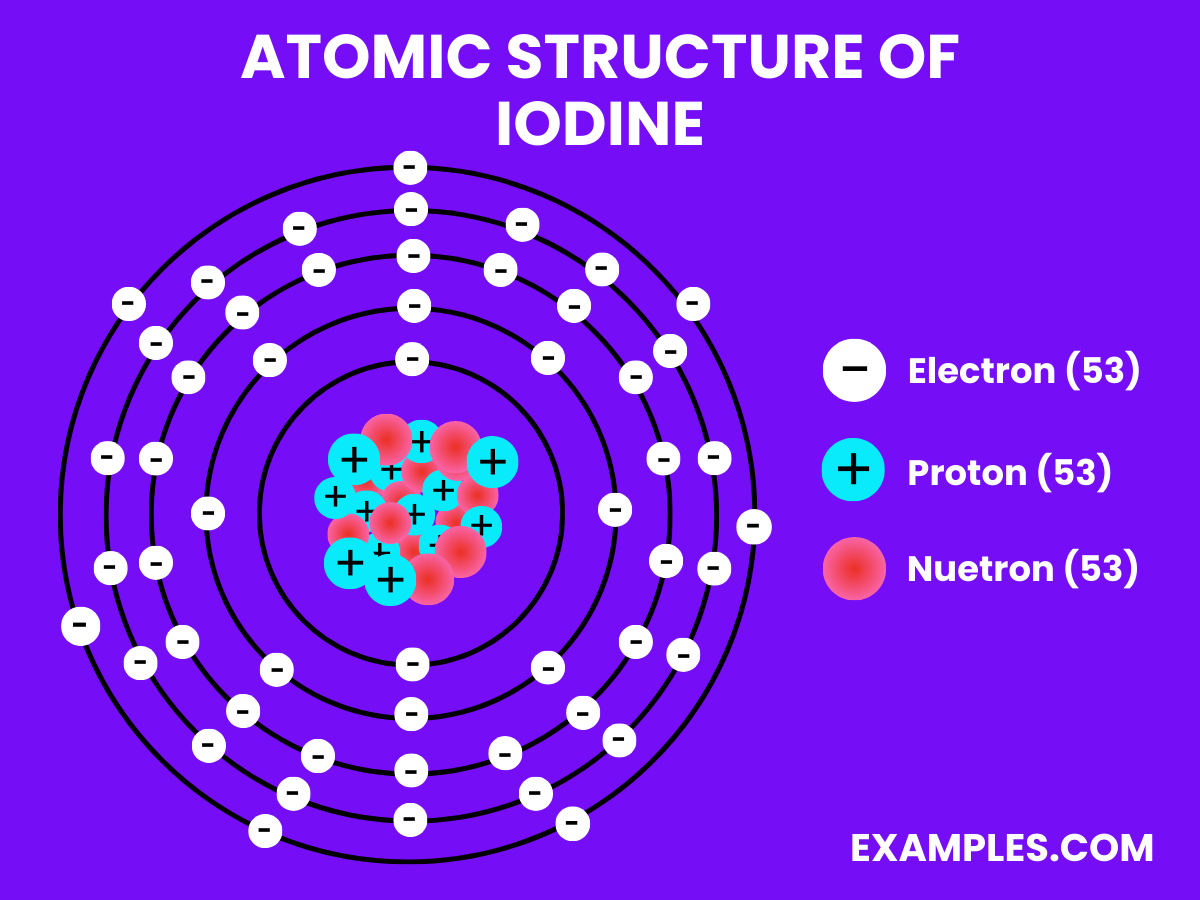
| Atomic Number | 53 |
| Atomic Mass | 126.90447 u |
| Neutron Cross Section | 6.15 barns (for ^127I) |
| Isotopes | Predominantly ^127I; Numerous radioactive isotopes, notably ^131I with a half-life of 8 days |
| Radioactivity | ^131I is commonly used in medical diagnostics and treatment, especially in thyroid conditions |
Iodine is a halogen element with a rich chemistry, primarily known for its roles in iodized salt and thyroid health. Here are some of the key compounds of iodine:
Iodine has several isotopes, but here are the most noteworthy, particularly Iodine-127 (the only stable isotope) and Iodine-131 (known for its medical applications):
| Isotope | Mass Number | Half-Life | Application or Note |
|---|---|---|---|
| I-123 | 123 | 13.22 hours | Used in nuclear medicine imaging, particularly for thyroid scans. |
| I-124 | 124 | 4.18 days | Useful in positron emission tomography (PET) imaging. |
| I-125 | 125 | 59.4 days | Widely used in medical diagnostics and treatment, especially in brachytherapy. |
| I-127 | 127 | Stable | The only stable isotope, natural iodine is composed of I-127. |
| I-129 | 129 | 15.7 million years | Long-lived radioactive isotope, used in geological dating. |
| I-131 | 131 | 8.02 days | Widely known for its use in treating thyroid cancer and hyperthyroidism. |
Iodine, a chemical element with the symbol I and atomic number 53, plays a vital role in various applications due to its unique properties. Here are some of its primary uses:
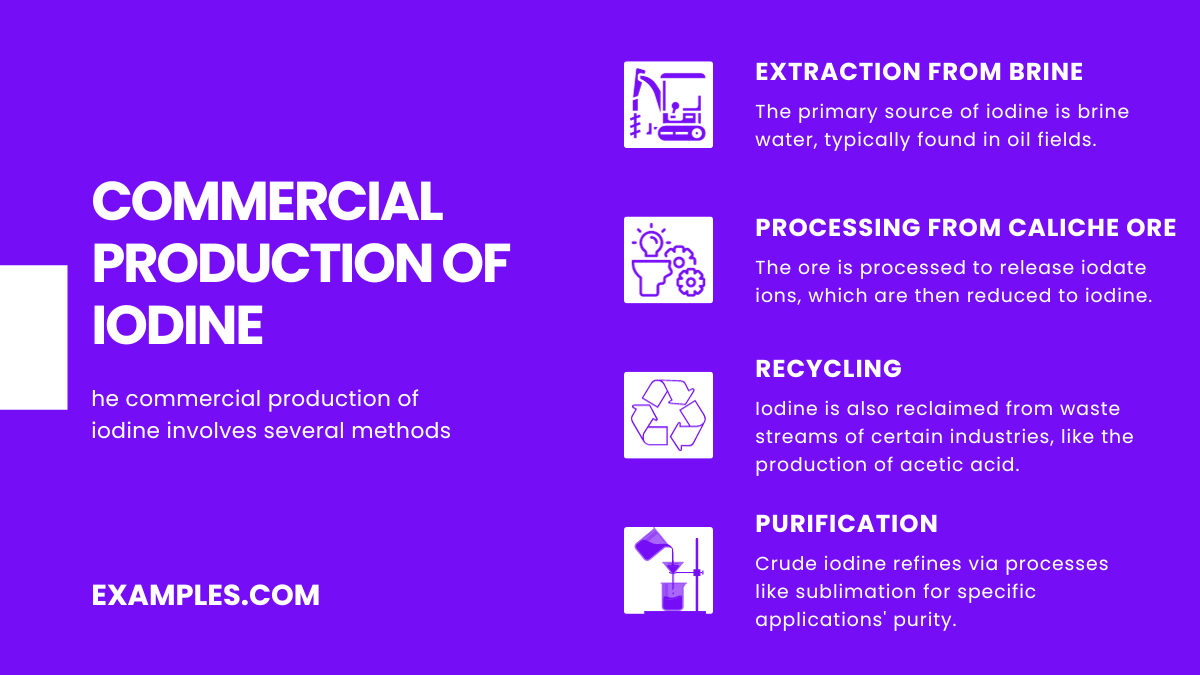
The commercial production of iodine involves several methods, primarily sourced from underground brines associated with natural gas and oil deposits. Here’s how iodine is typically produced:
In summary, iodine’s multifaceted uses in medical, industrial, and scientific domains highlight its importance. Its commercial production, primarily from brine sources, underscores a sophisticated process of extraction and purification to meet global demands.
Iodine is a vital micronutrient required for normal thyroid function, growth, and development. It’s found naturally in various foods and is also added to salt in many countries.
While iodine is essential for life, its distribution and availability in the environment can significantly impact ecosystems and human health.
Iodine is essential for thyroid hormone production, regulating metabolism, and supporting cognitive functions.
Seafood, dairy products, grains, and iodized salt are rich in iodine.
Iodine benefits include thyroid health, cognitive development, and preventing iodine deficiency disorders.
Taking iodine daily is safe in controlled amounts; consult healthcare providers for personal recommendations.
Iodine is in Group 17, the halogens, of the periodic table.
Iodine is an essential nutrient with significant health impacts. Regular consumption through iodine-rich foods ensures thyroid health and cognitive development. While daily intake is generally safe, it’s vital to adhere to recommended amounts. Understanding its role in chemistry as a halogen further highlights its importance. Always consult healthcare advice for personalized guidance, ensuring a healthy balance of this crucial element.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Iodine?
51
53
55
57
Which state of matter is iodine in at room temperature?
Gas
Liquid
Solid
Plasma
Iodine is necessary for the production of which type of hormones?
Adrenaline
Insulin
Thyroid
Estrogen
What color does iodine vapor exhibit?
Red
Blue
Violet
Green
Iodine deficiency can lead to which of the following health issues?
Diabetes
Goiter
Anemia
Rickets
Which compound is formed when iodine directly reacts with aluminum?
Aluminum oxide
Aluminum sulfide
Aluminum iodide
Aluminum nitrate
How does iodine act as an antiseptic?
By cooling the area
By dehydration
By oxidation
By reduction
What is the electron configuration of iodine's outer shell?
2, 8, 18, 18, 7
2, 8, 18, 17, 7
2, 8, 18, 18, 8
2, 8, 18, 18, 6
Which iodine isotope is used in medical imaging?
Iodine-123
Iodine-125
Iodine-131
Iodine-129
What is the primary source of iodine in the human diet?
Fresh fruits
Leafy greens
Seafood
Whole grains
Before you leave, take our quick quiz to enhance your learning!

