What is the atomic number of Bromine?
33
35
53
80
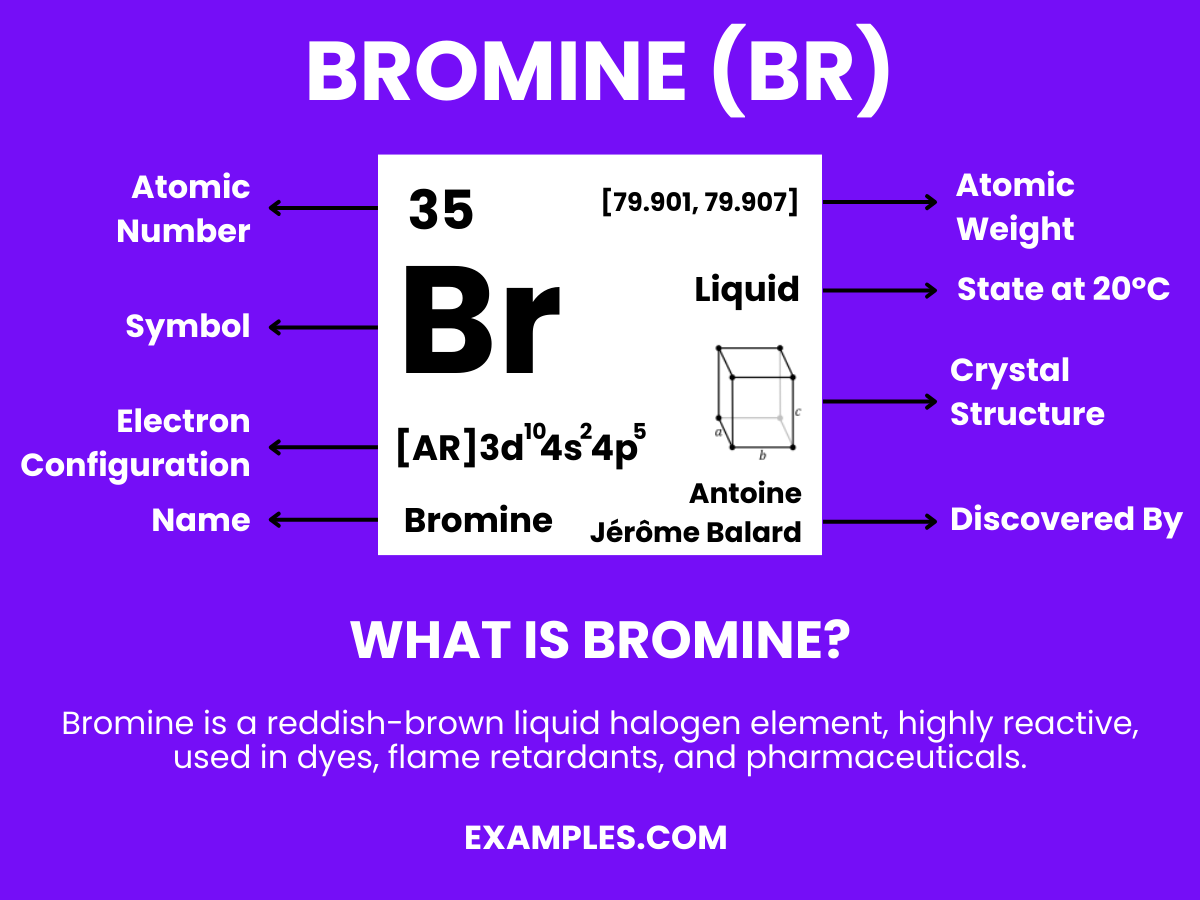
Bromine stands at the intersection of chemistry and practicality. In this comprehensive guide, we explore the deep red, volatile element known for its reactivity and versatility. Often paired with hydrogen to form hydrogen bromide, Bromine is pivotal in many industrial applications ranging from pharmaceuticals to flame retardants. This section is designed for educators aiming to enrich their teaching with vivid examples and real-world applications of Bromine, ensuring a captivating learning experience for students.
Bromine is a chemical element with the symbol Br and atomic number 35. It is a dark red, fuming liquid at room temperature and is one of only two elements that are liquid under normal conditions. Bromine is known for its sharp, pungent smell and is hazardous in its concentrated form. In simpler terms, it’s used in a variety of compounds and solutions, often seen in fire retardants, photography chemicals, and water purification processes. Understanding Bromine is fundamental in chemical education, offering insights into reactions, bonding, and the periodic table’s halogen group.
| Hydrogen | Phosphorus |
| Carbon | Sulfur |
| Nitrogen | Chlorine |
| Oxygen | Selenium |
| Fluorine | Iodine |
Formula: Br₂
Composition: Two bromine atoms.
Bond Type: A single covalent bond connecting the atoms.
Molecular Structure: Diatomic molecule.
Electron Configuration: Seven valence electrons per atom, fourteen in total for Br₂.
Significance: Widely used in flame retardants and certain types of medication.
Role in Chemistry: Used in the production of brominated organic compounds and photography chemicals. Commonly found in the form of liquid at room temperature.
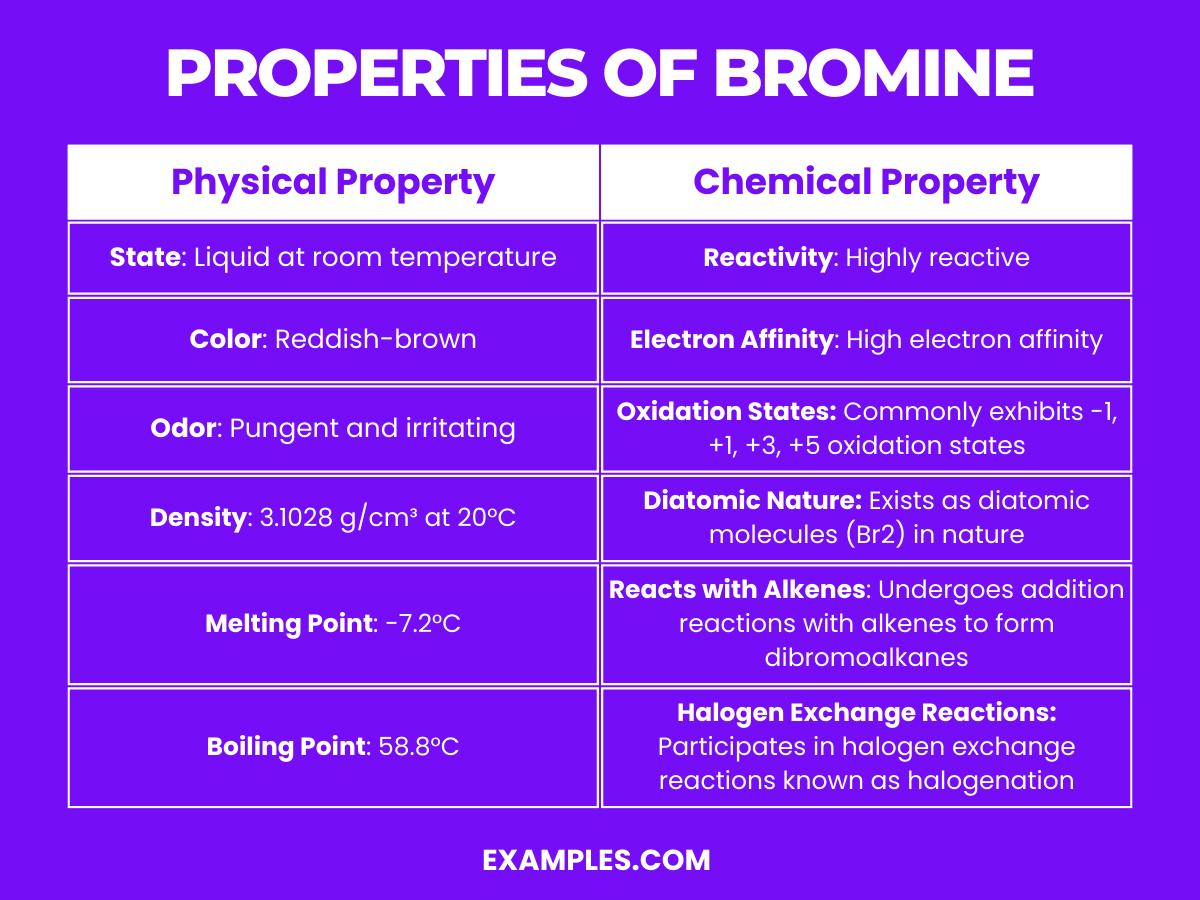
| Property | Description |
|---|---|
| State | Liquid at room temperature |
| Color | Reddish-brown |
| Odor | Pungent and irritating |
| Density | 3.1028 g/cm³ at 20°C |
| Melting Point | -7.2°C |
| Boiling Point | 58.8°C |
| Solubility | Soluble in organic solvents; slightly soluble in water |
| Atomic Mass | Approximately 79.904 u |
These physical properties define bromine’s distinct presence and behavior in various conditions and are essential in understanding how it can be stored, handled, and used in different applications.
Understanding the physical and chemical properties of bromine is crucial for its safe handling and utilization in various industrial and laboratory applications. Its high reactivity and versatile chemistry make it an important element in many chemical reactions and processes.
| Property | Value with Unit |
|---|---|
| Boiling Point | 58.8 °C |
| Melting Point | -7.3 °C |
| Critical Temperature | 320 °C |
| Critical Pressure | 10.3 MPa |
| Heat of Vaporization | 29.96 kJ/mol |
| Heat of Fusion | 10.57 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.474 J/g·K |
| Thermal Conductivity | 0.12 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 3.1028 g/cm³ (Liquid) |
| Viscosity (at 20°C) | 0.944 mPa·s (Liquid) |
| Solubility in Water (at 20°C) | 3.41 g/100 mL of water |
| Color | Reddish-brown |
| Phase at Room Temperature | Liquid |
| Property | Value with Unit |
|---|---|
| Electrical Conductivity | Non-conductive |
| Electronegativity (Pauling scale) | 2.96 |
| Ionization Energy | 11.81 eV |
| Electron Affinity | 3.36 eV |
| Property | Value with Unit |
|---|---|
| Atomic Number | 35 |
| Atomic Mass | 79.904 amu (Average) |
| Isotopes | ^79Br (50.69%), ^81Br (49.31%) |
| Nuclear Spin (for ^79Br) | 3/2 ℏ |
| Nuclear Spin (for ^81Br) | 3/2 ℏ |
| Neutron Cross Section (for ^79Br) | 6.8 barns |
| Neutron Cross Section (for ^81Br) | 2.4 barns |
| Nuclear Magnetic Moment (for ^79Br) | 2.106 µN |
| Nuclear Magnetic Moment (for ^81Br) | 2.224 µN |
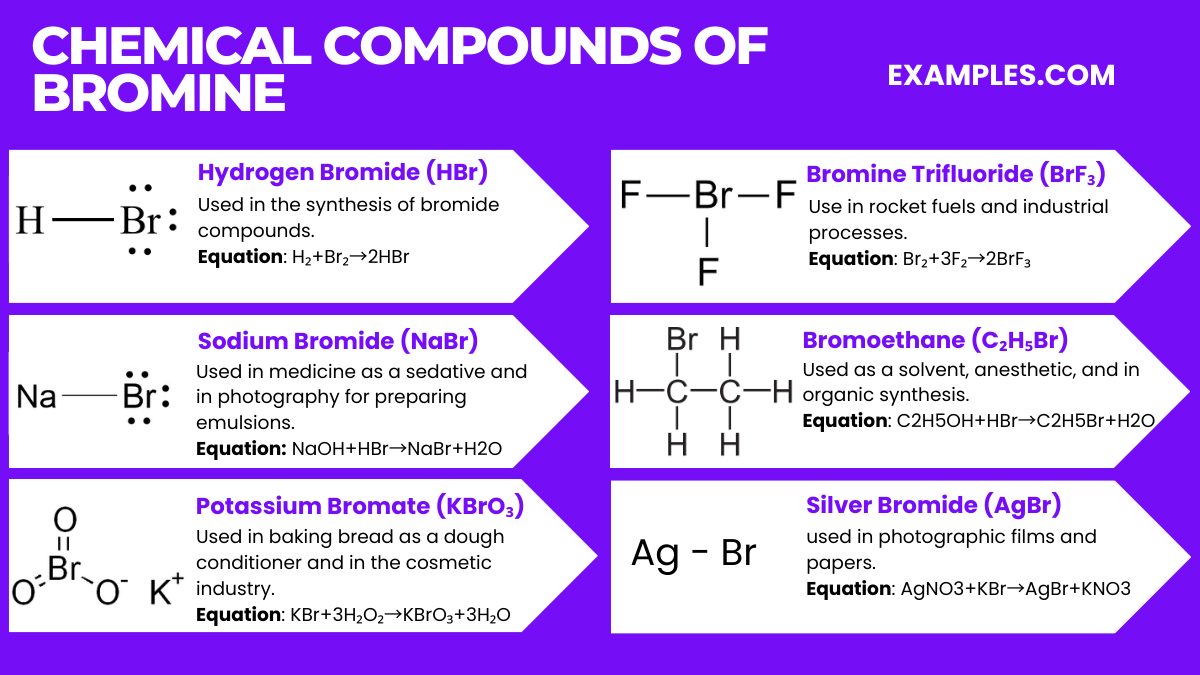
Bromine, a halogen element with the symbol Br, interacts with various elements and compounds to form a range of bromine compounds. Below are six compounds of bromine, along with their relevant chemical equations.
1. Hydrogen Bromide (HBr)
2. Sodium Bromide (NaBr)
3. Potassium Bromate (KBrO₃)
4. Bromine Trifluoride (BrF₃)
5. Bromoethane (C₂H₅Br)
6. Silver Bromide (AgBr)
Bromine has two stable isotopes which are present in nature: Bromine-79 and Bromine-81. Below is a table describing these isotopes:
| Isotope | Atomic Mass | Natural Abundance (%) | Half-Life | Nuclear Spin |
|---|---|---|---|---|
| Bromine-79 | 78.9183 amu | 50.69 | Stable | 3/2- |
| Bromine-81 | 80.9163 amu | 49.31 | Stable | 3/2- |
Both isotopes have a nuclear spin of 3/2-, making them useful for various nuclear magnetic resonance applications. Despite their differences in mass and abundance, both isotopes exhibit similar chemical behaviors, being indistinguishable in most chemical reactions.

Bromine, a halogen element with the symbol Br, is a volatile, reddish-brown liquid at room temperature. It has various applications in modern-day industries and science due to its unique properties. Here are some of its most prominent uses:
The commercial production of bromine predominantly occurs through the extraction from brine pools. Here is a detailed description of the process:
The bromine obtained through this process is then distributed for various industrial and scientific applications, forming an integral part of many manufacturing and production activities worldwide. The consistent demand across different sectors ensures the continued commercial production and use of bromine.
Bromine is a chemical element with the symbol Br and atomic number 35. It is a halogen, found primarily in the form of salts in sea water, brines, and salt lakes. Despite its natural occurrence, exposure to bromine in high concentrations can have various adverse health effects.
Bromine compounds, particularly brominated flame retardants and methyl bromide (used as a pesticide), have significant environmental impacts.
Bromine exposure can cause respiratory issues, skin irritation, and affect the nervous system, leading to various health problems.
Bromine is used in flame retardants, pesticides, pharmaceuticals, and photography chemicals due to its reactive nature.
High levels of bromine can lead to severe respiratory, skin, and neurological issues, making it harmful in concentrated exposures.
Bromine is used in flame retardants, photography chemicals, water purification, and as a pesticide. It’s essential in many industrial processes and consumer products.
While bromine is a naturally occurring element with various industrial uses, it poses significant health and environmental risks. Awareness and adherence to safety measures can mitigate these dangers. Understanding its effects and practicing responsible handling are crucial for protecting human health and maintaining ecological balance. Stay informed and cautious to navigate the world of bromine safely.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Bromine?
33
35
53
80
Bromine is one of the two elements in the periodic table that is:
Solid at room temperature
A noble gas
A liquid at room temperature
A metal
What type of bond does Bromine most commonly form?
Covalent
Ionic
Metallic
Hydrogen
Bromine is commonly used in the manufacture of:
Plastics
Pharmaceuticals
Flame retardants
Food additives
What is the primary health hazard associated with Bromine exposure?
Neurotoxicity
Dermatitis
Respiratory issues
Vision impairment
Which group of the periodic table does Bromine belong to?
Alkali metals
Alkaline earth metals
Halogens
Transition metals
Bromine naturally occurs in:
Free elemental form
Combined form in minerals
Atmospheric gases
Plant tissues
The color of bromine vapor is:
Colorless
Yellow
Reddish-brown
Blue
Bromine's name is derived from the Greek word 'bromos', which means:
Heavy
Stench
Bright
Bitter
Bromine reacts with aluminum to form:
Aluminum bromide
Aluminum oxide
Aluminum sulfate
Aluminum nitrate
Before you leave, take our quick quiz to enhance your learning!

