Understanding moles and molar mass is fundamental to mastering AP Chemistry. This topic is the cornerstone of stoichiometry, chemical reactions, and quantitative chemistry.
Learning Objectives:
By the end of this lesson, students should be able to define a mole and understand it as a unit of measurement using Avogadro’s number (6.022 x 10²³). They should be able to calculate molar mass and convert between mass, moles, and number of particles. Students will learn to define and calculate molarity, perform dilution calculations, and relate moles to chemical equations and stoichiometry, including identifying the limiting reactant and calculating theoretical yield. They should also be able to apply the ideal gas law and perform gas stoichiometry calculations. Finally, students will relate the concept of moles to real-world contexts, including laboratory experiments and practical applications in various fields. Key terms include mole, Avogadro’s number, molar mass, molarity, stoichiometry, limiting reactant, and ideal gas law.
The Mole Concept
What is Mole? – Definition
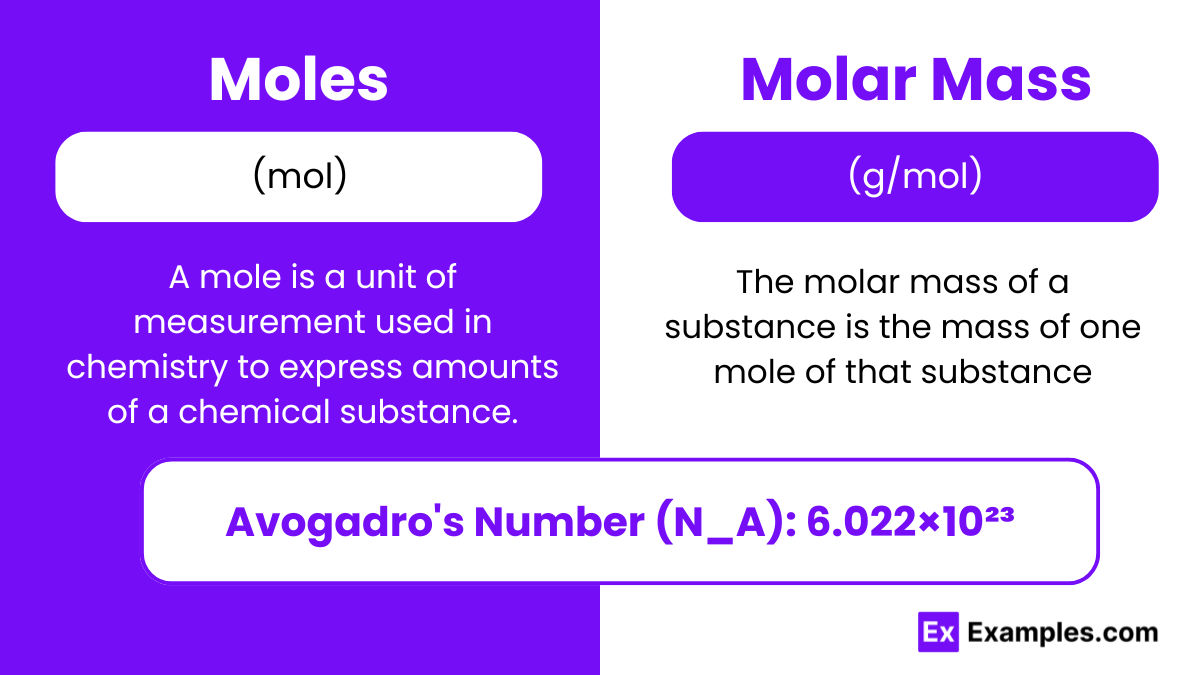
A mole is a unit of measurement used in chemistry to express amounts of a chemical substance. The mole (symbol: mol) is defined as the amount of any substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of pure carbon-12 (¹²C). This number is known as Avogadro’s number, which is approximately 6.022×1023.
Avogadro’s Number
Avogadro’s Number (N_A): 6.022×1023
- Represents the number of atoms, molecules, or particles in one mole of a substance.
- Essential for converting between atomic mass units (amu) and grams.
Importance of the Mole
- Provides a bridge between the atomic world (atoms and molecules) and the macroscopic world (grams and liters).
- Allows chemists to count particles by weighing them.
Molar Mass
What is Molar Mass? – Definition
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol). The molar mass of an element is numerically equal to its atomic mass in unified atomic mass units (u or amu).
How to Calculate Molar Mss
Here are the simple steps to calculate molar mass
- Identify the Elements
Determine which elements are in the compound and their quantities.
- Find Atomic Mass:
Look up the atomic masses of each element on the periodic table.
- Multiply and Sum
Multiply the atomic mass of each element by the number of atoms of that element in the molecule, then sum these values.
Example: Calculating the molar mass of H₂O
- Hydrogen (H): Atomic mass = 1.008 amu
- Oxygen (O): Atomic mass = 16.00 amu
Step 1: Molar mass of H₂O=(2×1.008 g/mol)+(1×16.00 g/mol)
Step 2: Molar mass of H₂O=2.016 g/mol+16.00 g/mol
Step 3: Molar mass of H₂O=18.016 g/mol
Common Molar Masses
Here are some common substances and their molar masses for quick reference:
- NaCl (Sodium Chloride): 58.44 g/mol
- H₂SO₄ (Sulfuric Acid): 98.08 g/mol
- C₆H₁₂O₆ (Glucose): 180.16 g/mol
- CO₂ (Carbon Dioxide): 44.01 g/mol
- NH₃ (Ammonia): 17.03 g/mol
- H₂O (Water): 18.016 g/mol
- O₂ (Oxygen Gas): 32.00 g/mol
- CH₄ (Methane): 16.04 g/mol
- C₂H₆ (Ethane): 30.07 g/mol
- C₃H₈ (Propane): 44.09 g/mol
- C₄H₁₀ (Butane): 58.12 g/mol
- C₂H₅OH (Ethanol): 46.07 g/mol
- HCl (Hydrochloric Acid): 36.46 g/mol
- NaOH (Sodium Hydroxide): 40.00 g/mol
- KOH (Potassium Hydroxide): 56.11 g/mol
- Ca(OH)₂ (Calcium Hydroxide): 74.10 g/mol
- Mg(OH)₂ (Magnesium Hydroxide): 58.32 g/mol
- NaHCO₃ (Sodium Bicarbonate): 84.01 g/mol
- CaCO₃ (Calcium Carbonate): 100.09 g/mol
- Na₂CO₃ (Sodium Carbonate): 105.99 g/mol
- H₂O₂ (Hydrogen Peroxide): 34.01 g/mol
- C₆H₆ (Benzene): 78.11 g/mol
- C₈H₁₀ (Toluene): 92.14 g/mol
- CH₃COOH (Acetic Acid): 60.05 g/mol
- NH₄Cl (Ammonium Chloride): 53.49 g/mol
- Fe₂O₃ (Iron(III) Oxide): 159.69 g/mol
- FeSO₄ (Iron(II) Sulfate): 151.91 g/mol
- CuSO₄ (Copper(II) Sulfate): 159.61 g/mol
- Al₂O₃ (Aluminum Oxide): 101.96 g/mol
- Pb(NO₃)₂ (Lead(II) Nitrate): 331.21 g/mol
- AgNO₃ (Silver Nitrate): 169.87 g/mol
- K₂SO₄ (Potassium Sulfate): 174.26 g/mol
- KCl (Potassium Chloride): 74.55 g/mol
- ZnSO₄ (Zinc Sulfate): 161.47 g/mol
- CaCl₂ (Calcium Chloride): 110.98 g/mol
Using Moles and Molar Mass in Calculations
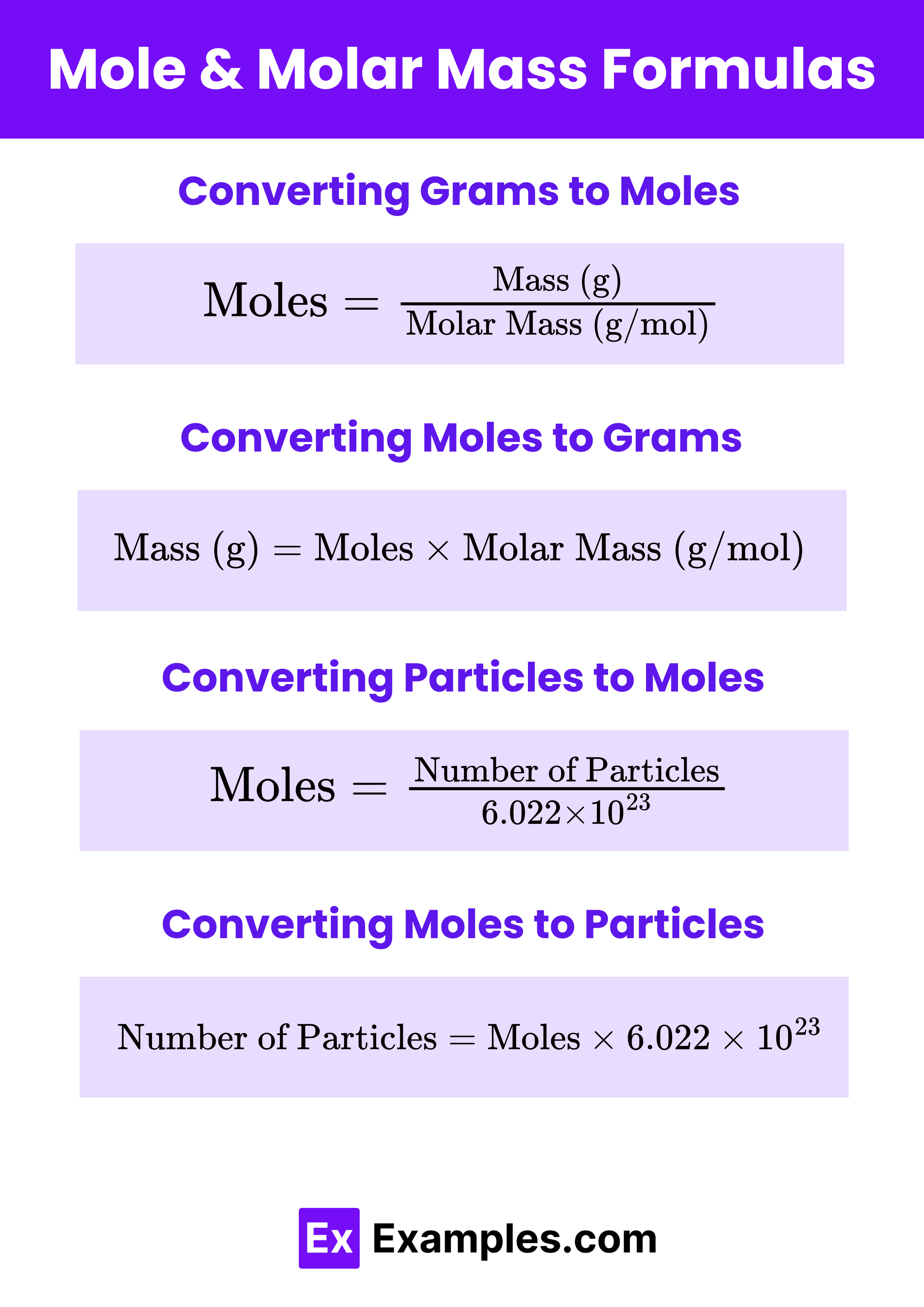
Converting Grams to Moles
To convert grams of a substance to moles, use the formula:
![]()
Example: Converting 36 grams of water (H₂O) to moles
![]()
Converting Moles to Grams
To convert moles of a substance to grams, use the formula:
![]()
Example: Converting 0.5 moles of water (H₂O) to grams
Mass of H₂O = 0.5 moles × 18.016 g/mol
Mass of H₂O ≈ 9.008 g
Converting Particles to Moles
To convert the number of particles (atoms, molecules, ions) to moles, use Avogadro’s number:
![]()
Example: Converting 1.204×1024 molecules of CO₂ to moles
![]()
Converting Moles to Particles
To convert moles to the number of particles, multiply by Avogadro’s number:
![]()
Number of Particles = Moles × 6.022×1023
Example: Converting 3 moles of H₂O to molecules
Number of Molecules of H₂O = 3 moles × 6.022 × 1023
![]()
Number of Molecules of H₂O = 1.807 × 1024 molecules
![]()